Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota
- PMID: 24475077
- PMCID: PMC3901675
- DOI: 10.1371/journal.pone.0086117
Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota
Abstract
Background and aim: Increasing evidence has indicated a close association of host-gut flora metabolic interaction with obesity. Flos Lonicera, a traditional herbal medicine, is used widely in eastern Asia for the treatment of various disorders. The aim of this study was to evaluate whether unfermented or fermented formulations of Flos Lonicera could exert a beneficial impact to combat obesity and related metabolic endotoxemia.
Methods: Obesity and metabolic endotoxemia were induced separately or together in rats through feeding a eight-week high fat diet either alone (HFD control group) or in combination with a single LPS stimulation (intraperitoneal injection, 0.75 mg/kg) (LPS control group). While, the mechanism of action of the Lonicera formulations was explored in vitro using RAW 264.7 and HCT 116 cell lines as models.
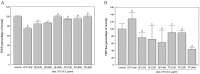
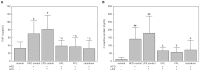
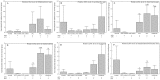
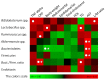
Results: In cell-based studies, treatment with both unfermented Flos Lonicera (UFL) and fermented Flos Lonicera (FFL) formulations resulted in suppression of LPS-induced NO production and gene expression of vital proinflammatory cytokines (TNF-α, COX-2, and IL-6) in RAW 264.7 cells, reduced the gene expression of zonula occludens (ZO)-1 and claudin-1, and normalized trans epithelial electric resistance (TEER) and horseradish peroxidase (HRP) flux in LPS-treated HCT-116 cells. In an animal study, treatment of HFD as well as HFD+LPS groups with UFL or FFL resulted in a notable decrease in body and adipose tissue weights, ameliorated total cholesterol, HDL, triglyceride, aspartate transaminase and endotoxin levels in serum, reduced the urinary lactulose/mannitol ratio, and markedly alleviated lipid accumulation in liver. In addition, exposure of HFD as well as HFD+LPS groups with UFL or FFL resulted in significant alteration of the distribution of intestinal flora, especially affecting the population of Akkermansia spp. and ratio of Bacteroidetes and Firmicutes.
Conclusion: This evidence collectively demonstrates that Flos Lonicera ameliorates obesity and related metabolic endotoxemia via regulating distribution of gut flora and gut permeability.
Conflict of interest statement
Figures






References
-
- Kopelman PG (2000) Obesity as a medical problem. Nature 404: 635–643. - PubMed
-
- National Institutes of Health (1998) Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda: National Institutes of Health.
-
- World Health Organization (2005) Preventing Chronic Diseases: a vital investment: WHO global report. Geneva: World Health Organization. 54–55 p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

