Farnesoid X receptor ligand prevents cisplatin-induced kidney injury by enhancing small heterodimer partner
- PMID: 24475141
- PMCID: PMC3903546
- DOI: 10.1371/journal.pone.0086553
Farnesoid X receptor ligand prevents cisplatin-induced kidney injury by enhancing small heterodimer partner
Abstract
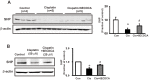
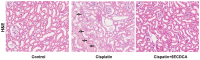
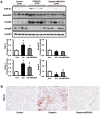
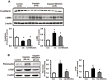
The farnesoid X receptor (FXR) is mainly expressed in liver, intestine and kidney. We investigated whether 6-ethyl chenodeoxycholic acid (6ECDCA), a semisynthetic derivative of chenodeoxycholic aicd (CDCA, an FXR ligand), protects against kidney injury and modulates small heterodimer partner (SHP) in cisplatin-induced kidney injury. Cisplatin inhibited SHP protein expression in the kidney of cisplatin-treated mice and human proximal tubular (HK2) cells; this effect was counteracted by FXR ligand. Hematoxylin and eosin staining revealed the presence of tubular casts, obstructions and dilatations in cisplatin-induced kidney injury, which was attenuated by FXR ligand. FXR ligand also attenuated protein expression of transforming growth factor-β1 (TGF-β1), Smad signaling, and the epithelial-to-mesenchymal transition process, inflammatory markers and cytokines, and apoptotic markers in cisplatin-treated mice. Cisplatin induced NF-κB activation in HK2 cell; this effect was attenuated by pretreatment with FXR ligand. In SHP knockdown by small interfering RNA, cisplatin-induced activation of TGF-β1, p-JNK and Bax/Bcl-2 ratio was not attenuated, while SHP overexpression and FXR ligand inhibited expression of these proteins in cisplatin-pretreated HK2 cells. In conclusion, FXR ligand, 6ECDCA prevents cisplatin-induced kidney injury, the underlying mechanism of which may be associated with anti-fibrotic, anti-inflammatory, and anti-apoptotic effects through SHP induction.
Conflict of interest statement
Figures





References
-
- Lebwohl D, Canetta R (1998) Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 34: 1522–1534. - PubMed
-
- Park JW, Cho JW, Joo SY, Kim CS, Choi JS, et al. (2012) Paricalcitol prevents cisplatin-induced renal injury by suppressing apoptosis and proliferation. Eur J Pharmacol 683: 301–309. - PubMed
-
- Yamate J, Tatsumi M, Nakatsuji S, Kuwamura M, Kotani T, et al. (1995) Immunohistochemical observations on the kinetics of macrophages and myofibroblasts in rat renal interstitial fibrosis induced by cis-diamminedichloroplatinum. J Comp Pathol 112: 27–39. - PubMed
-
- Yamate J, Machida Y, Ide M, Kuwamura M, Kotani T, et al. (2005) Cisplatin-induced renal interstitial fibrosis in neonatal rats, developing as solitary nephron unit lesions. Toxicol Pathol 33: 207–217. - PubMed
-
- Jiang M, Wei Q, Wang J, Du Q, Yu J, et al. (2006) Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

