Randomized controlled ferret study to assess the direct impact of 2008-09 trivalent inactivated influenza vaccine on A(H1N1)pdm09 disease risk
- PMID: 24475142
- PMCID: PMC3903544
- DOI: 10.1371/journal.pone.0086555
Randomized controlled ferret study to assess the direct impact of 2008-09 trivalent inactivated influenza vaccine on A(H1N1)pdm09 disease risk
Abstract
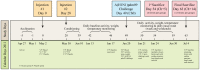
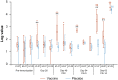
During spring-summer 2009, several observational studies from Canada showed increased risk of medically-attended, laboratory-confirmed A(H1N1)pdm09 illness among prior recipients of 2008-09 trivalent inactivated influenza vaccine (TIV). Explanatory hypotheses included direct and indirect vaccine effects. In a randomized placebo-controlled ferret study, we tested whether prior receipt of 2008-09 TIV may have directly influenced A(H1N1)pdm09 illness. Thirty-two ferrets (16/group) received 0.5 mL intra-muscular injections of the Canadian-manufactured, commercially-available, non-adjuvanted, split 2008-09 Fluviral or PBS placebo on days 0 and 28. On day 49 all animals were challenged (Ch0) with A(H1N1)pdm09. Four ferrets per group were randomly selected for sacrifice at day 5 post-challenge (Ch+5) and the rest followed until Ch+14. Sera were tested for antibody to vaccine antigens and A(H1N1)pdm09 by hemagglutination inhibition (HI), microneutralization (MN), nucleoprotein-based ELISA and HA1-based microarray assays. Clinical characteristics and nasal virus titers were recorded pre-challenge then post-challenge until sacrifice when lung virus titers, cytokines and inflammatory scores were determined. Baseline characteristics were similar between the two groups of influenza-naïve animals. Antibody rise to vaccine antigens was evident by ELISA and HA1-based microarray but not by HI or MN assays; virus challenge raised antibody to A(H1N1)pdm09 by all assays in both groups. Beginning at Ch+2, vaccinated animals experienced greater loss of appetite and weight than placebo animals, reaching the greatest between-group difference in weight loss relative to baseline at Ch+5 (7.4% vs. 5.2%; p = 0.01). At Ch+5 vaccinated animals had higher lung virus titers (log-mean 4.96 vs. 4.23pfu/mL, respectively; p = 0.01), lung inflammatory scores (5.8 vs. 2.1, respectively; p = 0.051) and cytokine levels (p>0.05). At Ch+14, both groups had recovered. Findings in influenza-naïve, systematically-infected ferrets may not replicate the human experience. While they cannot be considered conclusive to explain human observations, these ferret findings are consistent with direct, adverse effect of prior 2008-09 TIV receipt on A(H1N1)pdm09 illness. As such, they warrant further in-depth investigation and search for possible mechanistic explanations.
Conflict of interest statement
Figures



References
-
- Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, et al. (2010) Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: Four observational studies from Canada. PLoS Med 7(4): e1000258 10.1371/journal.pmed.1000258 - DOI - PMC - PubMed
-
- Tsuchihashi Y, Sunagawa T, Yahata Y, Takahashi H, Toyokawa T, et al. (2012) Association between seasonal influenza vaccination in 2008–2009 and pandemic influenza A (H1N1) 2009 infection among school students from Kobe, Japan, April-June 2009. Clin Infect Dis 54: 381–383. - PubMed
-
- Gilca R, Deceuninck G, De Serres G, Boulianne N, Sauvageau C, et al. (2011) Effectiveness of pandemic H1N1 vaccine against influenza-related hospitalization in children. Pediatrics 128: e1084–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

