Whole Pichia pastoris yeast expressing measles virus nucleoprotein as a production and delivery system to multimerize Plasmodium antigens
- PMID: 24475165
- PMCID: PMC3903550
- DOI: 10.1371/journal.pone.0086658
Whole Pichia pastoris yeast expressing measles virus nucleoprotein as a production and delivery system to multimerize Plasmodium antigens
Abstract
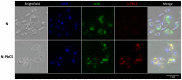
Yeasts are largely used as bioreactors for vaccine production. Usually, antigens are produced in yeast then purified and mixed with adjuvants before immunization. However, the purification costs and the safety concerns recently raised by the use of new adjuvants argue for alternative strategies. To this end, the use of whole yeast as both production and delivery system appears attractive. Here, we evaluated Pichia pastoris yeast as an alternative vaccine production and delivery system for the circumsporozoite protein (CS) of Plasmodium, the etiologic agent of malaria. The CS protein from Plasmodium berghei (Pb) was selected given the availability of the stringent C57Bl/6 mouse model of infection by Pb sporozoites, allowing the evaluation of vaccine efficacy in vivo. PbCS was multimerized by fusion to the measles virus (MV) nucleoprotein (N) known to auto-assemble in yeast in large-size ribonucleoprotein rods (RNPs). Expressed in P. pastoris, the N-PbCS protein generated highly multimeric and heterogenic RNPs bearing PbCS on their surface. Electron microscopy and immunofluorescence analyses revealed the shape of these RNPs and their localization in peripheral cytoplasmic inclusions. Subcutaneous immunization of C57Bl/6 mice with heat-inactivated whole P. pastoris expressing N-PbCS RNPs provided significant reduction of parasitemia after intradermal challenge with a high dose of parasites. Thus, in the absence of accessory adjuvants, a very low amount of PbCS expressed in whole yeast significantly decreased clinical damages associated with Pb infection in a highly stringent challenge model, providing a proof of concept of the intrinsic adjuvancy of this vaccine strategy. In addition to PbCS multimerization, the N protein contributed by itself to parasitemia delay and long-term mice survival. In the future, mixtures of whole recombinant yeasts expressing relevant Plasmodium antigens would provide a multivalent formulation applicable for antigen combination screening and possibly for large-scale production, distribution and delivery of a malaria vaccine in developing countries.
Conflict of interest statement
Figures



References
-
- FDA website. Common Ingredients in U.S. Licensed Vaccines. Vaccines, Blood & Biologics. Available:http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafe.... Accessed 2013 December 13.
-
- Ribeiro CM, Schijns VE (2010) Immunology of vaccine adjuvants. Methods Mol Biol 626: 1–14. - PubMed
-
- Bagnoli F, Baudner B, Mishra RP, Bartolini E, Fiaschi L, et al. (2011) Designing the next generation of vaccines for global public health. OMICS 15: 545–566. - PubMed
-
- Tomljenovic L (2011) Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis 23: 567–598. - PubMed
-
- Francois G, Duclos P, Margolis H, Lavanchy D, Siegrist CA, et al. (2005) Vaccine safety controversies and the future of vaccination programs. Pediatr Infect Dis J 24: 953–961. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

