Cell-type specific four-component hydrogel
- PMID: 24475174
- PMCID: PMC3903574
- DOI: 10.1371/journal.pone.0086740
Cell-type specific four-component hydrogel
Abstract
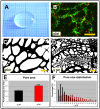
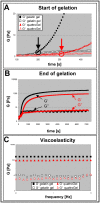
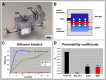
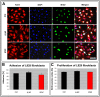
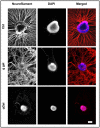
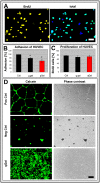
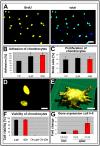
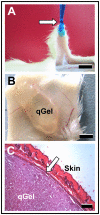
In the field of regenerative medicine we aim to develop implant matrices for specific tissue needs. By combining two per se, cell-permissive gel systems with enzymatic crosslinkers (gelatin/transglutaminase and fibrinogen/thrombin) to generate a blend (technical term: quattroGel), an unexpected cell-selectivity evolved. QuattroGels were porous and formed cavities in the cell diameter range, possessed gelation kinetics in the minute range, viscoelastic properties and a mechanical strength appropriate for general cell adhesion, and restricted diffusion. Cell proliferation of endothelial cells, chondrocytes and fibroblasts was essentially unaffected. In contrast, on quattroGels neither endothelial cells formed vascular tubes nor did primary neurons extend neurites in significant amounts. Only chondrocytes differentiated properly as judged by collagen isoform expression. The biophysical quattroGel characteristics appeared to leave distinct cell processes such as mitosis unaffected and favored differentiation of sessile cells, but hampered differentiation of migratory cells. This cell-type selectivity is of interest e.g. during articular cartilage or invertebral disc repair, where pathological innervation and angiogenesis represent adverse events in tissue engineering.
Conflict of interest statement
Figures
References
-
- Gibas I, Janik H (2010) Review: Synthetic polymer hydrogels for biomedical applications. Chemistry & Chemical Technology 4(4), 297–304.
-
- Seliktar D (2012) Designing cell-compatible hydrogels for biomedical applications. Science 336: 1124–1128. - PubMed
-
- Lum L, Elisseeff J (2003) Injectable Hydrogels for Cartilage Tissue Engineering. Topics in Tissue Engineering University of Oulu, Oulu 2003 Chapter 4.
-
- Fenglan X, Yubao L, Xuejiang W, Jie W, Aiping Y (2004) Preparation and characterization of nano-hydroxyapatite/poly(vinyl alcohol) hydrogel biocomposite. Journal of Materials Science 39: 5669–5672.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

