Sexual dimorphism in bite performance drives morphological variation in chameleons
- PMID: 24475183
- PMCID: PMC3903609
- DOI: 10.1371/journal.pone.0086846
Sexual dimorphism in bite performance drives morphological variation in chameleons
Abstract
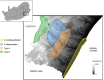
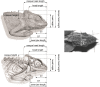
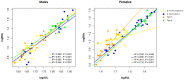
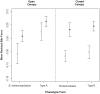
Phenotypic performance in different environments is central to understanding the evolutionary and ecological processes that drive adaptive divergence and, ultimately, speciation. Because habitat structure can affect an animal's foraging behaviour, anti-predator defences, and communication behaviour, it can influence both natural and sexual selection pressures. These selective pressures, in turn, act upon morphological traits to maximize an animal's performance. For performance traits involved in both social and ecological activities, such as bite force, natural and sexual selection often interact in complex ways, providing an opportunity to understand the adaptive significance of morphological variation with respect to habitat. Dwarf chameleons within the Bradypodion melanocephalum-Bradypodion thamnobates species complex have multiple phenotypic forms, each with a specific head morphology that could reflect its use of either open- or closed-canopy habitats. To determine whether these morphological differences represent adaptations to their habitats, we tested for differences in both absolute and relative bite performance. Only absolute differences were found between forms, with the closed-canopy forms biting harder than their open-canopy counterparts. In contrast, sexual dimorphism was found for both absolute and relative bite force, but the relative differences were limited to the closed-canopy forms. These results indicate that both natural and sexual selection are acting within both habitat types, but to varying degrees. Sexual selection seems to be the predominant force within the closed-canopy habitats, which are more protected from aerial predators, enabling chameleons to invest more in ornamentation for communication. In contrast, natural selection is likely to be the predominant force in the open-canopy habitats, inhibiting the development of conspicuous secondary sexual characteristics and, ultimately, enforcing their overall diminutive body size and constraining performance.
Conflict of interest statement
Figures


Similar articles
-
Sex-specific ecomorphological variation and the evolution of sexual dimorphism in dwarf chameleons (Bradypodion spp.).J Evol Biol. 2007 May;20(3):1073-81. doi: 10.1111/j.1420-9101.2007.01295.x. J Evol Biol. 2007. PMID: 17465917
-
Run for your life, but bite for your rights? How interactions between natural and sexual selection shape functional morphology across habitats.Naturwissenschaften. 2018 Jan 2;105(1-2):9. doi: 10.1007/s00114-017-1537-6. Naturwissenschaften. 2018. PMID: 29294185
-
Morphology, ornaments and performance in two chameleon ecomorphs: is the casque bigger than the bite?Zoology (Jena). 2009;112(3):217-26. doi: 10.1016/j.zool.2008.09.005. Epub 2009 Feb 20. Zoology (Jena). 2009. PMID: 19230632
-
Sexual selection, natural selection and the evolution of dimorphic coloration and ornamentation in agamid lizards.Proc Biol Sci. 2004 Nov 7;271(1554):2249-55. doi: 10.1098/rspb.2004.2802. Proc Biol Sci. 2004. PMID: 15539350 Free PMC article.
-
A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation.Biol Rev Camb Philos Soc. 2007 May;82(2):173-211. doi: 10.1111/j.1469-185X.2006.00004.x. Biol Rev Camb Philos Soc. 2007. PMID: 17437557 Review.
Cited by
-
From darkness to twilight: Morphological divergence between cave and surface-subterranean ecotone Niphargus species.Ecol Evol. 2024 Aug 6;14(8):e70061. doi: 10.1002/ece3.70061. eCollection 2024 Aug. Ecol Evol. 2024. PMID: 39108570 Free PMC article.
-
Universality of indeterminate growth in lizards rejected: the micro-CT reveals contrasting timing of growth cartilage persistence in iguanas, agamas, and chameleons.Sci Rep. 2019 Dec 12;9(1):18913. doi: 10.1038/s41598-019-54573-5. Sci Rep. 2019. PMID: 31831851 Free PMC article.
-
Off like a shot: scaling of ballistic tongue projection reveals extremely high performance in small chameleons.Sci Rep. 2016 Jan 4;6:18625. doi: 10.1038/srep18625. Sci Rep. 2016. PMID: 26725508 Free PMC article.
References
-
- Schluter D (2000) The Ecology of Adaptive Radiation; Harvey PH, May RM, editors. New York: Oxford University Press.
-
- Leal M, Fleishman LJ (2004) Differences in visual signal design and detectability between allopatric populations of Anolis lizards. American Naturalist 163: 26–39. - PubMed
-
- Herrel A, Spithoven L, Van Damme R, De Vree F (1999) Sexual dimorphism of head size in Gallotia galloti: testing the niche divergence hypothesis by functional analysis. Functional Ecology 13: 289–297.
-
- Measey GJ, Hopkins K, Tolley KA (2009) Morphology, ornaments and performance in two chameleon ecomorphs: is the casque bigger than the bite? Zoology 112: 217–226. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

