Inclusion of CD80 in HSV targets the recombinant virus to PD-L1 on DCs and allows productive infection and robust immune responses
- PMID: 24475315
- PMCID: PMC3903765
- DOI: 10.1371/journal.pone.0087617
Inclusion of CD80 in HSV targets the recombinant virus to PD-L1 on DCs and allows productive infection and robust immune responses
Abstract
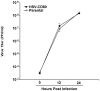
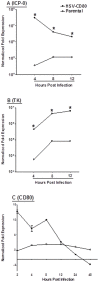
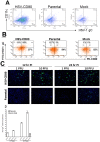
CD80 plays a critical role in stimulation of T cells and subsequent control of infection. To investigate the effect of CD80 on HSV-1 infection, we constructed a recombinant HSV-1 virus that expresses two copies of the CD80 gene in place of the latency associated transcript (LAT). This mutant virus (HSV-CD80) expressed high levels of CD80 and had similar virus replication kinetics as control viruses in rabbit skin cells. In contrast to parental virus, this CD80 expressing recombinant virus replicated efficiently in immature dendritic cells (DCs). Additionally, the susceptibility of immature DCs to HSV-CD80 infection was mediated by CD80 binding to PD-L1 on DCs. This interaction also contributed to a significant increase in T cell activation. Taken together, these results suggest that inclusion of CD80 as a vaccine adjuvant may promote increased vaccine efficacy by enhancing the immune response directly and also indirectly by targeting to DC.
Conflict of interest statement
Figures




Similar articles
-
Herpes Simplex Virus 1 ICP22 Suppresses CD80 Expression by Murine Dendritic Cells.J Virol. 2019 Jan 17;93(3):e01803-18. doi: 10.1128/JVI.01803-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30404803 Free PMC article.
-
Suppression of CD80 Expression by ICP22 Affects Herpes Simplex Virus Type 1 Replication and CD8+IFN-γ+ Infiltrates in the Eyes of Infected Mice but Not Latency Reactivation.J Virol. 2021 Sep 9;95(19):e0103621. doi: 10.1128/JVI.01036-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34287036 Free PMC article.
-
CD80 Plays a Critical Role in Increased Inflammatory Responses in Herpes Simplex Virus 1-Infected Mouse Corneas.J Virol. 2020 Jan 6;94(2):e01511-19. doi: 10.1128/JVI.01511-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31619558 Free PMC article.
-
Are miRNAs critical determinants in herpes simplex virus pathogenesis?Microbes Infect. 2018 Oct-Nov;20(9-10):461-465. doi: 10.1016/j.micinf.2017.12.007. Epub 2017 Dec 26. Microbes Infect. 2018. PMID: 29287990 Free PMC article. Review.
-
Vaccines for Herpes Simplex: Recent Progress Driven by Viral and Adjuvant Immunology.Methods Mol Biol. 2020;2060:31-56. doi: 10.1007/978-1-4939-9814-2_2. Methods Mol Biol. 2020. PMID: 31617171 Review.
Cited by
-
Varicella zoster virus productively infects human peripheral blood mononuclear cells to modulate expression of immunoinhibitory proteins and blocking PD-L1 enhances virus-specific CD8+ T cell effector function.PLoS Pathog. 2019 Mar 14;15(3):e1007650. doi: 10.1371/journal.ppat.1007650. eCollection 2019 Mar. PLoS Pathog. 2019. PMID: 30870532 Free PMC article.
-
Mutations within the pathogenic region of herpes simplex virus 1 gK signal sequences alter cell surface expression and neurovirulence.J Virol. 2015 Mar;89(5):2530-42. doi: 10.1128/JVI.03506-14. Epub 2014 Dec 10. J Virol. 2015. PMID: 25505072 Free PMC article.
-
Co-Delivery Effect of CD24 on the Immunogenicity and Lethal Challenge Protection of a DNA Vector Expressing Nucleocapsid Protein of Crimean Congo Hemorrhagic Fever Virus.Viruses. 2019 Jan 17;11(1):75. doi: 10.3390/v11010075. Viruses. 2019. PMID: 30658445 Free PMC article.
-
CD8+ T Cells Play a Bystander Role in Mice Latently Infected with Herpes Simplex Virus 1.J Virol. 2016 Apr 29;90(10):5059-5067. doi: 10.1128/JVI.00255-16. Print 2016 May 15. J Virol. 2016. PMID: 26962220 Free PMC article.
-
Loss of ICP22 in HSV-1 Elicits Immune Infiltration and Maintains Stromal Keratitis Despite Reduced Primary and Latent Virus Infectivity.Invest Ophthalmol Vis Sci. 2019 Aug 1;60(10):3398-3406. doi: 10.1167/iovs.19-27701. Invest Ophthalmol Vis Sci. 2019. PMID: 31387116 Free PMC article.
References
-
- Pozzi LA, Maciaszek JW, Rock KL (2005) Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J Immunol 175: 2071–2081. - PubMed
-
- van den Broeke LT, Daschbach E, Thomas EK, Andringa G, Berzofsky JA (2003) Dendritic cell-induced activation of adaptive and innate antitumor immunity. J Immunol 171: 5842–5852. - PubMed
-
- Colonna M, Pulendran B, Iwasaki A (2006) Dendritic cells at the host-pathogen interface. Nature Immunology 7: 117–120. - PubMed
-
- Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106: 255–258. - PubMed
-
- Steinman RM, Witmer MD, Nussenzweig MC, Chen LL, Schlesinger S, et al. (1980) Dendritic cells of the mouse: identification and characterization. J Invest Dermatol 75: 14–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY013191/EY/NEI NIH HHS/United States
- R01 EY013191/EY/NEI NIH HHS/United States
- R01 EY022695/EY/NEI NIH HHS/United States
- AI093941/AI/NIAID NIH HHS/United States
- EY13615/EY/NEI NIH HHS/United States
- L30 EY021919/EY/NEI NIH HHS/United States
- P01 AI078897/AI/NIAID NIH HHS/United States
- 5P01 AI078897/AI/NIAID NIH HHS/United States
- K08 EY020575/EY/NEI NIH HHS/United States
- R21 AI093941/AI/NIAID NIH HHS/United States
- R01 EY013615/EY/NEI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

