Larval body patterning and apical organs are conserved in animal evolution
- PMID: 24476105
- PMCID: PMC3939940
- DOI: 10.1186/1741-7007-12-7
Larval body patterning and apical organs are conserved in animal evolution
Abstract
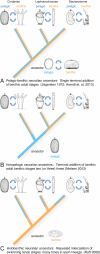
Background: Planktonic ciliated larvae are characteristic for the life cycle of marine invertebrates. Their most prominent feature is the apical organ harboring sensory cells and neurons of largely undetermined function. An elucidation of the relationships between various forms of primary larvae and apical organs is key to understanding the evolution of animal life cycles. These relationships have remained enigmatic due to the scarcity of comparative molecular data.
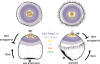
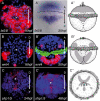
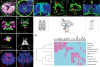
Results: To compare apical organs and larval body patterning, we have studied regionalization of the episphere, the upper hemisphere of the trochophore larva of the marine annelid Platynereis dumerilii. We examined the spatial distribution of transcription factors and of Wnt signaling components previously implicated in anterior neural development. Pharmacological activation of Wnt signaling with Gsk3β antagonists abolishes expression of apical markers, consistent with a repressive role of Wnt signaling in the specification of apical tissue. We refer to this Wnt-sensitive, six3- and foxq2-expressing part of the episphere as the 'apical plate'. We also unraveled a molecular signature of the apical organ--devoid of six3 but expressing foxj, irx, nkx3 and hox--that is shared with other marine phyla including cnidarians. Finally, we characterized the cell types that form part of the apical organ by profiling by image registration, which allows parallel expression profiling of multiple cells. Besides the hox-expressing apical tuft cells, this revealed the presence of putative light- and mechanosensory as well as multiple peptidergic cell types that we compared to apical organ cell types of other animal phyla.
Conclusions: The similar formation of a six3+, foxq2+ apical plate, sensitive to Wnt activity and with an apical tuft in its six3-free center, is most parsimoniously explained by evolutionary conservation. We propose that a simple apical organ--comprising an apical tuft and a basal plexus innervated by sensory-neurosecretory apical plate cells--was present in the last common ancestors of cnidarians and bilaterians. One of its ancient functions would have been the control of metamorphosis. Various types of apical plate cells would then have subsequently been added to the apical organ in the divergent bilaterian lineages. Our findings support an ancient and common origin of primary ciliated larvae.
Figures



Similar articles
-
An anterior signaling center patterns and sizes the anterior neuroectoderm of the sea urchin embryo.Development. 2016 May 1;143(9):1523-33. doi: 10.1242/dev.128165. Epub 2016 Mar 7. Development. 2016. PMID: 26952978 Free PMC article.
-
Development of the larval anterior neurogenic domains of Terebratalia transversa (Brachiopoda) provides insights into the diversification of larval apical organs and the spiralian nervous system.Evodevo. 2012 Jan 24;3:3. doi: 10.1186/2041-9139-3-3. Evodevo. 2012. PMID: 22273002 Free PMC article.
-
Expression of the wnt gene complement in a spiral-cleaving embryo and trochophore larva.Int J Dev Biol. 2014;58(6-8):563-73. doi: 10.1387/ijdb.140084ss. Int J Dev Biol. 2014. PMID: 25690970
-
Towards a systems-level understanding of development in the marine annelid Platynereis dumerilii.Curr Opin Genet Dev. 2016 Aug;39:175-181. doi: 10.1016/j.gde.2016.07.005. Epub 2016 Aug 6. Curr Opin Genet Dev. 2016. PMID: 27501412 Review.
-
Evo-Devo of Urbilateria and its larval forms.Dev Biol. 2022 Jul;487:10-20. doi: 10.1016/j.ydbio.2022.04.003. Epub 2022 Apr 17. Dev Biol. 2022. PMID: 35443190 Review.
Cited by
-
Gene activation of metazoan Fox transcription factors at the onset of metamorphosis in the marine demosponge Amphimedon queenslandica.Dev Growth Differ. 2022 Oct;64(8):455-468. doi: 10.1111/dgd.12812. Dev Growth Differ. 2022. PMID: 36155915 Free PMC article.
-
Diversity of cilia-based mechanosensory systems and their functions in marine animal behaviour.Philos Trans R Soc Lond B Biol Sci. 2020 Feb 17;375(1792):20190376. doi: 10.1098/rstb.2019.0376. Epub 2019 Dec 30. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31884914 Free PMC article. Review.
-
Identification of neural transcription factors required for the differentiation of three neuronal subtypes in the sea urchin embryo.Dev Biol. 2018 Mar 15;435(2):138-149. doi: 10.1016/j.ydbio.2017.12.015. Epub 2018 Jan 10. Dev Biol. 2018. PMID: 29331498 Free PMC article.
-
From nerve net to nerve ring, nerve cord and brain--evolution of the nervous system.Nat Rev Neurosci. 2016 Jan;17(1):61-72. doi: 10.1038/nrn.2015.15. Nat Rev Neurosci. 2016. PMID: 26675821 Review.
-
Neuronal patterning of the tubular collar cord is highly conserved among enteropneusts but dissimilar to the chordate neural tube.Sci Rep. 2017 Aug 1;7(1):7003. doi: 10.1038/s41598-017-07052-8. Sci Rep. 2017. PMID: 28765531 Free PMC article.
References
-
- Young CM, Sewell MA. Atlas of Marine Invertebrate Larvae. Waltham, MA, USA: Academic Press; 2006.
-
- Nielsen C. Trochophora larvae: cell-lineages, ciliary bands and body regions. 2. Other groups and general discussion. J Exp Zool B Mol Dev Evol. 2005;12:401–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

