Azithromycin may antagonize inhaled tobramycin when targeting Pseudomonas aeruginosa in cystic fibrosis
- PMID: 24476418
- PMCID: PMC4028742
- DOI: 10.1513/AnnalsATS.201310-352OC
Azithromycin may antagonize inhaled tobramycin when targeting Pseudomonas aeruginosa in cystic fibrosis
Abstract
Rationale: Recent studies of inhaled tobramycin in subjects with cystic fibrosis (CF) find less clinical improvement than previously observed. Nonhuman data suggest that in some strains of Pseudomonas aeruginosa, azithromycin can antagonize tobramycin.
Objectives: We tested the hypothesis that concomitant azithromycin use correlates with less improvement in key outcome measures in subjects receiving inhaled tobramycin while not affecting those receiving a comparative, nonaminoglycoside inhaled antibiotic.
Methods: We studied a cohort of 263 subjects with CF enrolled in a recent clinical trial comparing inhaled tobramycin with aztreonam lysine. We performed a secondary analysis to examine key clinical and microbiologic outcomes based on concomitant, chronic azithromycin use at enrollment.
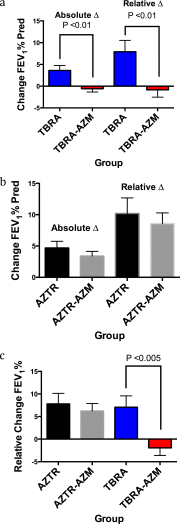
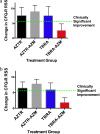
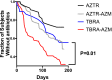
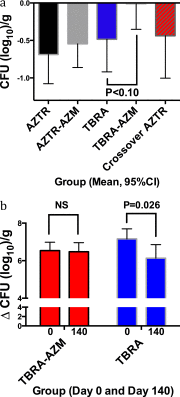
Measurements and main results: The cohort randomized to inhaled tobramycin and reporting azithromycin use showed a significant decrease in the percent predicted FEV1 after one and three courses of inhaled tobramycin when compared with those not reporting azithromycin use (28 d: -0.51 vs. 3.43%, P < 0.01; 140 d: -1.87 vs. 6.07%, P < 0.01). Combined azithromycin and inhaled tobramycin use was also associated with earlier need for additional antibiotics, lesser improvement in disease-related quality of life, and a trend toward less reduction in sputum P. aeruginosa density. Subjects randomized to inhaled aztreonam lysine had significantly greater improvement in these outcome measures, which were unaffected by concomitant azithromycin use. Outcomes in those not using azithromycin who received inhaled tobramycin were not significantly different from subjects receiving aztreonam lysine. Azithromycin also antagonized tobramycin but not aztreonam lysine in 40% of P. aeruginosa clinical isolates tested in vitro.
Conclusions: Oral azithromycin may antagonize the therapeutic benefits of inhaled tobramycin in subjects with CF with P. aeruginosa airway infection.
Figures
Comment in
-
Practice guidelines, clinical trials, and unexpected results in cystic fibrosis.Ann Am Thorac Soc. 2014 Mar;11(3):402-3. doi: 10.1513/AnnalsATS.201401-023ED. Ann Am Thorac Soc. 2014. PMID: 24673695 No abstract available.
References
-
- Cystic Fibrosis Foundation Patient Registry 2011 Annual Data Report Bethesda, MD: 2012[accessed 1 Aug 2013]. Available from: http://www.cff.org/UploadedFiles/research/ClinicalResearch/2011-Patient-...
-
- Courtney JM, Bradley J, Mccaughan J, O’Connor TM, Shortt C, Bredin CP, Bradbury I, Elborn JS. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol. 2007;42:525–532. - PubMed
-
- Lopes AJ, Mafort TT, de Sá Ferreira A, Santos de Castro MC, Cássia de Firmida M, de Andrade Marques E. Is the type of chronic pulmonary infection a determinant of lung function outcomes in adult patients with cystic fibrosis? Monaldi Arch Chest Dis. 2012;77:122–128. - PubMed
-
- Assael BM, Pressler T, Bilton D, Fayon M, Fischer R, Chiron R, Larosa M, Knoop C, McElvaney N, Lewis SA, et al. For the AZLI Active Comparator Study Group. Inhaled aztreonam lysine vs. inhaled tobramycin in cystic fibrosis: a comparative efficacy trial. J Cyst Fibros. 2012;12:130–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

