Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling
- PMID: 24476943
- PMCID: PMC4023150
- DOI: 10.1038/npp.2014.24
Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling
Abstract
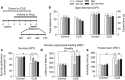
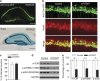
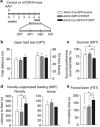
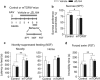
The endocannabinoid (eCB) system regulates mood, emotion, and stress coping, and dysregulation of the eCB system is critically involved in pathophysiology of depression. The eCB ligand 2-arachidonoylglycerol (2-AG) is inactivated by monoacylglycerol lipase (MAGL). Using chronic unpredictable mild stress (CUS) as a mouse model of depression, we examined how 2-AG signaling in the hippocampus was altered in depressive-like states and how this alteration contributed to depressive-like behavior. We report that CUS led to impairment of depolarization-induced suppression of inhibition (DSI) in mouse hippocampal CA1 pyramidal neurons, and this deficiency in 2-AG-mediated retrograde synaptic depression was rescued by MAGL inhibitor JZL184. CUS induced depressive-like behaviors and decreased mammalian target of rapamycin (mTOR) activation in the hippocampus, and these biochemical and behavioral abnormalities were ameliorated by chronic JZL184 treatments. The effects of JZL184 were mediated by cannabinoid CB1 receptors. Genetic deletion of mTOR with adeno-associated viral (AAV) vector carrying the Cre recombinase in the hippocampus of mTORf/f mice recapitulated depressive-like behaviors induced by CUS and abrogated the antidepressant-like effects of chronic JZL184 treatments. Our results suggest that CUS decreases eCB-mTOR signaling in the hippocampus, leading to depressive-like behaviors, whereas MAGL inhibitor JZL184 produces antidepressant-like effects through enhancement of eCB-mTOR signaling.
Figures




References
-
- Bambico FR, Duranti A, Tontini A, Tarzia G, Gobbi G. Endocannabinoids in the treatment of mood disorders: evidence from animal models. Curr Pharm Des. 2009;15:1623–1646. - PubMed
-
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–117. - PubMed
-
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

