Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat
- PMID: 24477073
- PMCID: PMC3952927
- DOI: 10.5966/sctm.2013-0112
Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat
Abstract
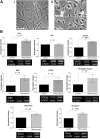
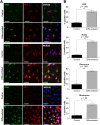
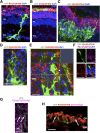
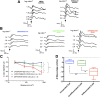
Müller glia possess stem cell characteristics that have been recognized to be responsible for the regeneration of injured retina in fish and amphibians. Although these cells are present in the adult human eye, they are not known to regenerate human retina in vivo. Human Müller glia with stem cell characteristics (hMSCs) can acquire phenotypic and genotypic characteristics of rod photoreceptors in vitro, suggesting that they may have potential for use in transplantation strategies to treat human photoreceptor degenerations. Much work has been undertaken in rodents using various sources of allogeneic stem cells to restore photoreceptor function, but the effect of human Müller glia-derived photoreceptors in the restoration of rod photoreceptor function has not been investigated. This study aimed to differentiate hMSCs into photoreceptor cells by stimulation with growth and differentiation factors in vitro to upregulate gene and protein expression of CRX, NR2E3, and rhodopsin and various phototransduction markers associated with rod photoreceptor development and function and to examine the effect of subretinal transplantation of these cells into the P23H rat, a model of primary photoreceptor degeneration. Following transplantation, hMSC-derived photoreceptor cells migrated and integrated into the outer nuclear layer of the degenerated retinas and led to significant improvement in rod photoreceptor function as shown by an increase in a-wave amplitude and slope using scotopic flash electroretinography. These observations suggest that hMSCs can be regarded as a cell source for development of cell-replacement therapies to treat human photoreceptor degenerations and may also offer potential for the development of autologous transplantation.
Keywords: Müller glia; Photoreceptors; Repair and regeneration; Retina; Stem cells; Transplantation.
Figures
References
-
- Meyer RL. Evidence from thymidine labeling for continuing growth of retina and tectum in juvenile goldfish. Exp Neurol. 1978;59:99–111. - PubMed
-
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. - PubMed
-
- Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Müller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. - PubMed
-
- Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. - PubMed
-
- Bhatia B, Singhal S, Lawrence JM, et al. Distribution of Müller stem cells within the neural retina: Evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp Eye Res. 2009;89:373–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

