Arthritic periosteal tissue from joint replacement surgery: a novel, autologous source of stem cells
- PMID: 24477075
- PMCID: PMC3952924
- DOI: 10.5966/sctm.2013-0056
Arthritic periosteal tissue from joint replacement surgery: a novel, autologous source of stem cells
Abstract
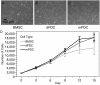
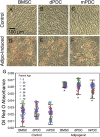
The overarching aim of this study is to assess the feasibility of using periosteal tissue from the femoral neck of arthritic hip joints, usually discarded in the normal course of hip replacement surgery, as an autologous source of stem cells. In addition, the study aims to characterize intrinsic differences between periosteum-derived cell (PDC) populations, isolated via either enzymatic digestion or a migration assay, including their proliferative capacity, surface marker expression, and multipotency, relative to commercially available human bone marrow-derived stromal cells (BMSCs) cultured under identical conditions. Commercial BMSCs and PDCs were characterized in vitro, using a growth assay, flow cytometry, as well as assay of Oil Red O, alizarin red, and Safranin O/Fast Green staining after respective culture in adipo-, osteo-, and chondrogenic media. Based on these outcome measures, PDCs exhibited proliferation rate, morphology, surface receptor expression, and multipotency similar to those of BMSCs. No significant correlation was observed between outcome measures and donor age or diagnosis (osteoarthritis [OA] and rheumatoid arthritis [RA], respectively), a profound finding given recent rheumatological studies indicating that OA and RA share not only common biomarkers and molecular mechanisms but also common pathophysiology, ultimately resulting in the need for joint replacement. Furthermore, PDCs isolated via enzymatic digestion and migration assay showed subtle differences in surface marker expression but otherwise no significant differences in proliferation or multipotency; the observed differences in surface marker expression may indicate potential effects of isolation method on the population of cells isolated and/or the behavior of the respective isolated cell populations. This study demonstrates, for the first time to our knowledge, the feasibility of using arthritic tissue resected during hip replacement as a source of autologous stem cells. In sum, periosteum tissue that is resected with the femoral neck in replacing the hip represents an unprecedented and, to date, unstudied source of stem cells from OA and RA patients. Follow-up studies will determine the degree to which this new, autologous source of stem cells can be banked for future use.
Keywords: Arthritis; Clinical translation; Differentiation; Osteoarthritis; Periosteum; Regenerative medicine; Rheumatoid arthritis; Stem cell.
Figures


References
-
- Badowski MS, Harris DT. Collection, processing, and banking of umbilical cord blood stem cells for transplantation and regenerative medicine. Methods Mol Biol. 2012;879:279–290. - PubMed
-
- Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18:453–463. - PubMed
-
- Dhanasekaran M, Indumathi S, Poojitha R, et al. Plasticity and banking potential of cultured adipose tissue derived mesenchymal stem cells. Cell Tissue Bank. 2013;14:303–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

