Phase-dependent resetting of the adrenal clock by ACTH in vitro
- PMID: 24477539
- PMCID: PMC3949112
- DOI: 10.1152/ajpregu.00519.2013
Phase-dependent resetting of the adrenal clock by ACTH in vitro
Abstract
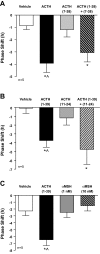
The adrenal cortex has a molecular clock that generates circadian rhythms in glucocorticoids, yet how the clock is synchronized to the external environment is unknown. Using mPER2::Luciferase (mPER2Luc) knockin mice, in which luciferase is rhythmically expressed under the control of the mouse Per2 clock gene, we hypothesized that ACTH transmits entrainment signals to the adrenal. Adrenal explants were administered ACTH at different phases of the mPER2Luc rhythm. Treatment with ACTH 1-39 produced a phase delay that was phase-dependent, with a maximum at circadian time (CT)18; ACTH did not alter the period or amplitude of the rhythm. Forskolin produced a parallel response, suggesting that the phase delay was cAMP-mediated. The response to ACTH was concentration-dependent and peptide-specific. Pulse administration (60 min) of ACTH 1-39 also produced phase delays restricted to late CTs. In contrast to ACTH 1-39, other ACTH fragments, including α-melanocyte-stimulating hormone, which do not activate the melanocortin 2 (MC2/ACTH) receptor, had no effect. The finding that ACTH in vitro phase delays the adrenal mPER2luc rhythm in a monophasic fashion argues for ACTH as a key resetter, but not the sole entrainer, of the adrenal clock.
Keywords: adrenal clock; adrenocorticotropic hormone; nonphotic entrainment.
Figures




References
-
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000 - PubMed
-
- Birmingham MK, Kurlents E. Inactivation of ACTH by isolated rat adrenals and inhibition of corticoid formation by adrenocortical hormones. Endocrinology 62: 47–60, 1962 - PubMed
-
- Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse adrenal tissues. Am J Physiol Regul Integr Comp Physiol 285: R561–R569, 2003 - PubMed
-
- Buijs RM, Scheer FA, Kreier F, Yi C, Bos N, Goncharuk VD, Kalsbeek A. Organization of circadian functions: interaction with the body. Prog Brain Res 153: 341–360, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

