Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism
- PMID: 24477690
- PMCID: PMC3926057
- DOI: 10.1073/pnas.1316001111
Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism
Abstract
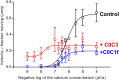
Myosin-binding protein C (MyBP-C) is an accessory protein of striated muscle thick filaments and a modulator of cardiac muscle contraction. Defects in the cardiac isoform, cMyBP-C, cause heart disease. cMyBP-C includes 11 Ig- and fibronectin-like domains and a cMyBP-C-specific motif. In vitro studies show that in addition to binding to the thick filament via its C-terminal region, cMyBP-C can also interact with actin via its N-terminal domains, modulating thin filament motility. Structural observations of F-actin decorated with N-terminal fragments of cMyBP-C suggest that cMyBP-C binds to actin close to the low Ca(2+) binding site of tropomyosin. This suggests that cMyBP-C might modulate thin filament activity by interfering with tropomyosin regulatory movements on actin. To determine directly whether cMyBP-C binding affects tropomyosin position, we have used electron microscopy and in vitro motility assays to study the structural and functional effects of N-terminal fragments binding to thin filaments. 3D reconstructions suggest that under low Ca(2+) conditions, cMyBP-C displaces tropomyosin toward its high Ca(2+) position, and that this movement corresponds to thin filament activation in the motility assay. At high Ca(2+), cMyBP-C had little effect on tropomyosin position and caused slowing of thin filament sliding. Unexpectedly, a shorter N-terminal fragment did not displace tropomyosin or activate the thin filament at low Ca(2+) but slowed thin filament sliding as much as the larger fragments. These results suggest that cMyBP-C may both modulate thin filament activity, by physically displacing tropomyosin from its low Ca(2+) position on actin, and govern contractile speed by an independent molecular mechanism.
Keywords: muscle activation; muscle regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extraction, purification and characterization. J Mol Biol. 1973;74(4):653–676. - PubMed
-
- Winegrad S. Cardiac myosin binding protein C. Circ Res. 1999;84(10):1117–1126. - PubMed
-
- Craig R, Offer G. The location of C-protein in rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1976;192(1109):451–461. - PubMed
-
- Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: Its role in physiology and disease. Circ Res. 2004;94(10):1279–1289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

