Immunogenicity of a prime-boost vaccine containing the circumsporozoite proteins of Plasmodium vivax in rodents
- PMID: 24478093
- PMCID: PMC3911365
- DOI: 10.1128/IAI.01410-13
Immunogenicity of a prime-boost vaccine containing the circumsporozoite proteins of Plasmodium vivax in rodents
Expression of concern in
-
Expression of Concern for Teixeira et al., "Immunogenicity of a Prime-Boost Vaccine Containing the Circumsporozoite Proteins of Plasmodium vivax in Rodents".Infect Immun. 2025 Feb 18;93(2):e0054424. doi: 10.1128/iai.00544-24. Epub 2025 Jan 13. Infect Immun. 2025. PMID: 39804089 Free PMC article. No abstract available.
Abstract
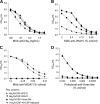
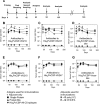
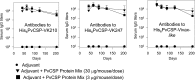
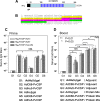
Plasmodium vivax is the most widespread and the second most prevalent malaria-causing species in the world. Current measures used to control the transmission of this disease would benefit from the development of an efficacious vaccine. In the case of the deadly parasite P. falciparum, the recombinant RTS,S vaccine containing the circumsporozoite antigen (CSP) consistently protects 30 to 50% of human volunteers against infection and is undergoing phase III clinical trials in Africa with similar efficacy. These findings encouraged us to develop a P. vivax vaccine containing the three circulating allelic forms of P. vivax CSP. Toward this goal, we generated three recombinant bacterial proteins representing the CSP alleles, as well as a hybrid polypeptide called PvCSP-All-CSP-epitopes. This hybrid contains the conserved N and C termini of P. vivax CSP and the three variant repeat domains in tandem. We also generated simian and human recombinant replication-defective adenovirus vectors expressing PvCSP-All-CSP-epitopes. Mice immunized with the mixture of recombinant proteins in a formulation containing the adjuvant poly(I·C) developed high and long-lasting serum IgG titers comparable to those elicited by proteins emulsified in complete Freund's adjuvant. Antibody titers were similar in mice immunized with homologous (protein-protein) and heterologous (adenovirus-protein) vaccine regimens. The antibodies recognized the three allelic forms of CSP, reacted to the repeated and nonrepeated regions of CSP, and recognized sporozoites expressing the alleles VK210 and VK247. The vaccine formulations described in this work should be useful for the further development of an anti-P. vivax vaccine.
Figures





Similar articles
-
Immunogenicity of recombinant proteins consisting of Plasmodium vivax circumsporozoite protein allelic variant-derived epitopes fused with Salmonella enterica Serovar Typhimurium flagellin.Clin Vaccine Immunol. 2013 Sep;20(9):1418-25. doi: 10.1128/CVI.00312-13. Epub 2013 Jul 17. Clin Vaccine Immunol. 2013. PMID: 23863502 Free PMC article.
-
A universal vaccine candidate against Plasmodium vivax malaria confers protective immunity against the three PvCSP alleles.Sci Rep. 2021 Sep 9;11(1):17928. doi: 10.1038/s41598-021-96986-1. Sci Rep. 2021. PMID: 34504134 Free PMC article.
-
Prime-boost vaccination with recombinant protein and adenovirus-vector expressing Plasmodium vivax circumsporozoite protein (CSP) partially protects mice against Pb/Pv sporozoite challenge.Sci Rep. 2018 Jan 18;8(1):1118. doi: 10.1038/s41598-017-19063-6. Sci Rep. 2018. PMID: 29348479 Free PMC article.
-
The march toward malaria vaccines.Vaccine. 2015 Nov 27;33 Suppl 4(Suppl 4):D13-23. doi: 10.1016/j.vaccine.2015.07.091. Epub 2015 Aug 29. Vaccine. 2015. PMID: 26324116 Free PMC article. Review.
-
The March Toward Malaria Vaccines.Am J Prev Med. 2015 Dec;49(6 Suppl 4):S319-33. doi: 10.1016/j.amepre.2015.09.011. Am J Prev Med. 2015. PMID: 26590432 Free PMC article. Review.
Cited by
-
Heterologous vaccination utilizing viral vector and protein platforms confers complete protection against SFTSV.Sci Rep. 2023 May 20;13(1):8189. doi: 10.1038/s41598-023-35328-9. Sci Rep. 2023. PMID: 37210393 Free PMC article.
-
Structural basis of Plasmodium vivax inhibition by antibodies binding to the circumsporozoite protein repeats.Elife. 2022 Jan 13;11:e72908. doi: 10.7554/eLife.72908. Elife. 2022. PMID: 35023832 Free PMC article.
-
Tissue signatures influence the activation of intrahepatic CD8(+) T cells against malaria sporozoites.Front Microbiol. 2014 Aug 22;5:440. doi: 10.3389/fmicb.2014.00440. eCollection 2014. Front Microbiol. 2014. PMID: 25202304 Free PMC article. Review.
-
Current Progress of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) Vaccine Development.Viruses. 2024 Jan 16;16(1):128. doi: 10.3390/v16010128. Viruses. 2024. PMID: 38257828 Free PMC article. Review.
-
A Multistage Formulation Based on Full-Length CSP and AMA-1 Ectodomain of Plasmodium vivax Induces High Antibody Titers and T-cells and Partially Protects Mice Challenged with a Transgenic Plasmodium berghei Parasite.Microorganisms. 2020 Jun 17;8(6):916. doi: 10.3390/microorganisms8060916. Microorganisms. 2020. PMID: 32560380 Free PMC article.
References
-
- Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kaboré W, Ouédraogo S, Sandrine Y, Guiguemdé RT, Ouédraogo JB, Hamel MJ, Kariuki S, Odero C, Oneko M, Otieno K, Awino N, Omoto J, Williamson J, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Nekoye O, Gondi S, Otieno A, Ogutu B, Wasuna R, Owira V, Jones D, Onyango AA, Njuguna P, Chilengi R, Akoo P, Kerubo C, Gitaka J, Maingi C, Lang T, Olotu A, Tsofa B, Bejon P, Peshu N, Marsh K, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Ayamba S, Kayan K, Owusu-Ofori R, Dosoo D, Asante I, Adjei G, Adjei G, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Kilavo H, Mahende C, Liheluka E, Lemnge M, Theander T, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Agyekum A, Owusu L, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Msika A, Jumbe A, Chome N, Nyakuipa D, Chintedza J, Ballou WR, Bruls M, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Vekemans J, Carter T, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P, RTS,S Clinical Trials Partnership 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365:1863–1875. 10.1056/NEJMoa1102287 - DOI - PubMed
-
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl. Trop. Dis. 4:e774. 10.1371/journal.pntd.0000774 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

