Antibodies to a single, conserved epitope in Anopheles APN1 inhibit universal transmission of Plasmodium falciparum and Plasmodium vivax malaria
- PMID: 24478095
- PMCID: PMC3911399
- DOI: 10.1128/IAI.01222-13
Antibodies to a single, conserved epitope in Anopheles APN1 inhibit universal transmission of Plasmodium falciparum and Plasmodium vivax malaria
Abstract
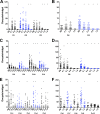
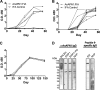
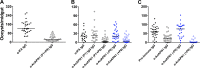
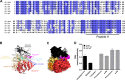
Malaria transmission-blocking vaccines (TBVs) represent a promising approach for the elimination and eradication of this disease. AnAPN1 is a lead TBV candidate that targets a surface antigen on the midgut of the obligate vector of the Plasmodium parasite, the Anopheles mosquito. In this study, we demonstrated that antibodies targeting AnAPN1 block transmission of Plasmodium falciparum and Plasmodium vivax across distantly related anopheline species in countries to which malaria is endemic. Using a biochemical and immunological approach, we determined that the mechanism of action for this phenomenon stems from antibody recognition of a single protective epitope on AnAPN1, which we found to be immunogenic in murine and nonhuman primate models and highly conserved among anophelines. These data indicate that AnAPN1 meets the established target product profile for TBVs and suggest a potential key role for an AnAPN1-based panmalaria TBV in the effort to eradicate malaria.
Figures


References
-
- World Health Organization 2012. World Malaria Report. World Health Organization, Geneva, Switzerland
-
- Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M. 2011. A research agenda to underpin malaria eradication. PLoS Med. 8:e1000406. 10.1371/journal.pmed.1000406 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

