Neonatal infraorbital nerve crush-induced CNS synaptic plasticity and functional recovery
- PMID: 24478162
- PMCID: PMC4035773
- DOI: 10.1152/jn.00658.2013
Neonatal infraorbital nerve crush-induced CNS synaptic plasticity and functional recovery
Abstract
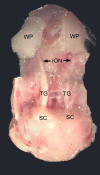
Infraorbital nerve (ION) transection in neonatal rats leads to disruption of whisker-specific neural patterns (barrelettes), conversion of functional synapses into silent synapses, and reactive gliosis in the brain stem trigeminal principal nucleus (PrV). Here we tested the hypothesis that neonatal peripheral nerve crush injuries permit better functional recovery of associated central nervous system (CNS) synaptic circuitry compared with nerve transection. We developed an in vitro whisker pad-trigeminal ganglion (TG)-brain stem preparation in neonatal rats and tested functional recovery in the PrV following ION crush. Intracellular recordings revealed that 68% of TG cells innervate the whisker pad. We used the proportion of whisker pad-innervating TG cells as an index of ION function. The ION function was blocked by ∼64%, immediately after mechanical crush, then it recovered beginning after 3 days postinjury and was complete by 7 days. We used this reversible nerve-injury model to study peripheral nerve injury-induced CNS synaptic plasticity. In the PrV, the incidence of silent synapses increased to ∼3.5 times of control value by 2-3 days postinjury and decreased to control levels by 5-7 days postinjury. Peripheral nerve injury-induced reaction of astrocytes and microglia in the PrV was also reversible. Neonatal ION crush disrupted barrelette formation, and functional recovery was not accompanied by de novo barrelette formation, most likely due to occurrence of recovery postcritical period (P3) for pattern formation. Our results suggest that nerve crush is more permissive for successful regeneration and reconnection (collectively referred to as "recovery" here) of the sensory inputs between the periphery and the brain stem.
Keywords: brain stem; nerve damage; rat; silent synapses; trigeminal.
Figures




Similar articles
-
Neonatal sensory nerve injury-induced synaptic plasticity in the trigeminal principal sensory nucleus.Exp Neurol. 2016 Jan;275 Pt 2(0 2):245-52. doi: 10.1016/j.expneurol.2015.04.022. Epub 2015 May 6. Exp Neurol. 2016. PMID: 25956829 Free PMC article. Review.
-
Sensory Activity-Dependent and Sensory Activity-Independent Properties of the Developing Rodent Trigeminal Principal Nucleus.Dev Neurosci. 2016;38(3):163-170. doi: 10.1159/000446395. Epub 2016 Jun 9. Dev Neurosci. 2016. PMID: 27287019 Free PMC article. Review.
-
Comparative study of the neuronal plasticity along the neuraxis of the vibrissal sensory system of adult rat following unilateral infraorbital nerve damage and subsequent regeneration.Exp Brain Res. 1999 May;126(2):259-69. doi: 10.1007/s002210050735. Exp Brain Res. 1999. PMID: 10369148
-
Astrocytes promote peripheral nerve injury-induced reactive synaptogenesis in the neonatal CNS.J Neurophysiol. 2011 Dec;106(6):2876-87. doi: 10.1152/jn.00312.2011. Epub 2011 Sep 7. J Neurophysiol. 2011. PMID: 21900512 Free PMC article.
-
Neonatal deafferentation does not alter membrane properties of trigeminal nucleus principalis neurons.J Neurophysiol. 2001 Mar;85(3):1088-96. doi: 10.1152/jn.2001.85.3.1088. J Neurophysiol. 2001. PMID: 11247979 Free PMC article.
Cited by
-
Neonatal sensory nerve injury-induced synaptic plasticity in the trigeminal principal sensory nucleus.Exp Neurol. 2016 Jan;275 Pt 2(0 2):245-52. doi: 10.1016/j.expneurol.2015.04.022. Epub 2015 May 6. Exp Neurol. 2016. PMID: 25956829 Free PMC article. Review.
-
Enhancement of median nerve regeneration by mesenchymal stem cells engraftment in an absorbable conduit: improvement of peripheral nerve morphology with enlargement of somatosensory cortical representation.Front Neuroanat. 2014 Oct 16;8:111. doi: 10.3389/fnana.2014.00111. eCollection 2014. Front Neuroanat. 2014. PMID: 25360086 Free PMC article.
-
Early recovery of neuronal functioning in the sensory cortex after nerve reconstruction surgery.Restor Neurol Neurosci. 2019;37(4):409-419. doi: 10.3233/RNN-190914. Restor Neurol Neurosci. 2019. PMID: 31322584 Free PMC article.
-
Sensory Activity-Dependent and Sensory Activity-Independent Properties of the Developing Rodent Trigeminal Principal Nucleus.Dev Neurosci. 2016;38(3):163-170. doi: 10.1159/000446395. Epub 2016 Jun 9. Dev Neurosci. 2016. PMID: 27287019 Free PMC article. Review.
References
-
- Alcalá-Galiano A, Arribas-García IJ, Martín-Pérez MA, Romance A, Montalvo-Moreno JJ, Juncos JM. Pediatric facial fractures: children are not just small adults. Radiographics 28: 441–461, 2008 - PubMed
-
- Aldskogius H. Mechanisms and consequences of microglial responses to peripheral axotomy. Front Biosci 3: 857–868, 2011 - PubMed
-
- Baumgartner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136: 147–167, 2013 - PubMed
-
- Bates CA, Erzurumlu RS, Killackey HP. Central correlates of peripheral pattern alterations in the trigeminal system of the rat. III. Neurons of the principal sensory nucleus. Brain Res 281: 108–113, 1982 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

