Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement
- PMID: 24478281
- PMCID: PMC3928932
- DOI: 10.1098/rsif.2013.0962
Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement
Abstract
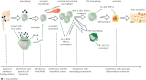
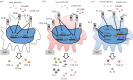
Wear particles and by-products from joint replacements and other orthopaedic implants may result in a local chronic inflammatory and foreign body reaction. This may lead to persistent synovitis resulting in joint pain and swelling, periprosthetic osteolysis, implant loosening and pathologic fracture. Strategies to modulate the adverse effects of wear debris may improve the function and longevity of joint replacements and other orthopaedic implants, potentially delaying or avoiding complex revision surgical procedures. Three novel biological strategies to mitigate the chronic inflammatory reaction to orthopaedic wear particles are reported. These include (i) interference with systemic macrophage trafficking to the local implant site, (ii) modulation of macrophages from an M1 (pro-inflammatory) to an M2 (anti-inflammatory, pro-tissue healing) phenotype in the periprosthetic tissues, and (iii) local inhibition of the transcription factor nuclear factor kappa B (NF-κB) by delivery of an NF-κB decoy oligodeoxynucleotide, thereby interfering with the production of pro-inflammatory mediators. These three approaches have been shown to be viable strategies for mitigating the undesirable effects of wear particles in preclinical studies. Targeted local delivery of specific biologics may potentially extend the lifetime of orthopaedic implants.
Keywords: NF-κB; cell trafficking; inflammation; macrophage polarization; orthopaedic implants; wear particles.
Figures

References
-
- Jacobs JJ, Shanbhag A, Glant TT, Black J, Galante JO. 1994. Wear debris in total joint replacements. J. Am. Acad. Orthop. Surg. 2, 212–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
