Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1
- PMID: 24478369
- PMCID: PMC3905150
- DOI: 10.1523/JNEUROSCI.4043-13.2014
Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1
Abstract
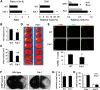
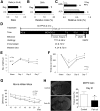
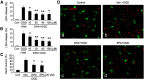
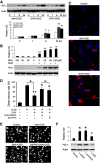
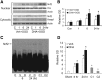
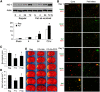
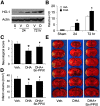
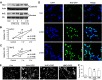
Ischemic stroke is a debilitating clinical disorder that affects millions of people, yet lacks effective neuroprotective treatments. Fish oil is known to exert beneficial effects against cerebral ischemia. However, the underlying protective mechanisms are not fully understood. The present study tests the hypothesis that omega-3 polyunsaturated fatty acids (n-3 PUFAs) attenuate ischemic neuronal injury by activating nuclear factor E2-related factor 2 (Nrf2) and upregulating heme oxygenase-1 (HO-1) in both in vitro and in vivo models. We observed that pretreatment of rat primary neurons with docosahexaenoic acid (DHA) significantly reduced neuronal death following oxygen-glucose deprivation. This protection was associated with increased Nrf2 activation and HO-1 upregulation. Inhibition of HO-1 activity with tin protoporphyrin IX attenuated the protective effects of DHA. Further studies showed that 4-hydroxy-2E-hexenal (4-HHE), an end-product of peroxidation of n-3 PUFAs, was a more potent Nrf2 inducer than 4-hydroxy-2E-nonenal derived from n-6 PUFAs. In an in vivo setting, transgenic mice overexpressing fatty acid metabolism-1, an enzyme that converts n-6 PUFAs to n-3 PUFAs, were remarkably resistant to focal cerebral ischemia compared with their wild-type littermates. Regular mice fed with a fish oil-enhanced diet also demonstrated significant resistance to ischemia compared with mice fed with a regular diet. As expected, the protection was associated with HO-1 upregulation, Nrf2 activation, and 4-HHE generation. Together, our data demonstrate that n-3 PUFAs are highly effective in protecting the brain, and that the protective mechanisms involve Nrf2 activation and HO-1 upregulation by 4-HHE. Further investigation of n-3 PUFA neuroprotective mechanisms may accelerate the development of stroke therapies.
Figures

Similar articles
-
4-Hydroxy hexenal derived from docosahexaenoic acid protects endothelial cells via Nrf2 activation.PLoS One. 2013 Jul 23;8(7):e69415. doi: 10.1371/journal.pone.0069415. Print 2013. PLoS One. 2013. PMID: 23936010 Free PMC article.
-
4-Hydroxy hexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs.Biochem Biophys Res Commun. 2014 Jan 17;443(3):991-6. doi: 10.1016/j.bbrc.2013.12.085. Epub 2013 Dec 19. Biochem Biophys Res Commun. 2014. PMID: 24361890
-
Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells.Biochem Biophys Res Commun. 2010 Nov 5;402(1):99-104. doi: 10.1016/j.bbrc.2010.09.124. Epub 2010 Oct 12. Biochem Biophys Res Commun. 2010. PMID: 20920477
-
Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders.Mol Neurobiol. 2011 Oct;44(2):192-201. doi: 10.1007/s12035-011-8181-5. Epub 2011 Apr 19. Mol Neurobiol. 2011. PMID: 21499987 Free PMC article. Review.
-
Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases.Prostaglandins Leukot Essent Fatty Acids. 2018 Sep;136:3-13. doi: 10.1016/j.plefa.2017.03.006. Epub 2017 Mar 10. Prostaglandins Leukot Essent Fatty Acids. 2018. PMID: 28314621 Free PMC article. Review.
Cited by
-
Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke.Exp Neurol. 2015 Oct;272:170-80. doi: 10.1016/j.expneurol.2015.03.005. Epub 2015 Mar 12. Exp Neurol. 2015. PMID: 25771800 Free PMC article.
-
Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders-A Review.Nutrients. 2018 Oct 21;10(10):1561. doi: 10.3390/nu10101561. Nutrients. 2018. PMID: 30347877 Free PMC article. Review.
-
The cell-specific roles of Nrf2 in acute and chronic phases of ischemic stroke.CNS Neurosci Ther. 2024 Mar;30(3):e14462. doi: 10.1111/cns.14462. Epub 2023 Sep 16. CNS Neurosci Ther. 2024. PMID: 37715557 Free PMC article. Review.
-
Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-operative Pain After Bone Fracture in Mice.Front Pharmacol. 2018 May 1;9:412. doi: 10.3389/fphar.2018.00412. eCollection 2018. Front Pharmacol. 2018. PMID: 29765320 Free PMC article.
-
Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells.J Neuroinflammation. 2018 Jul 9;15(1):202. doi: 10.1186/s12974-018-1232-3. J Neuroinflammation. 2018. PMID: 29986724 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
