Tissue plasminogen activator contributes to alterations of neuronal migration and activity-dependent responses in fragile X mice
- PMID: 24478370
- PMCID: PMC6827590
- DOI: 10.1523/JNEUROSCI.3753-13.2014
Tissue plasminogen activator contributes to alterations of neuronal migration and activity-dependent responses in fragile X mice
Abstract
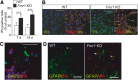
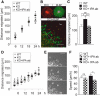
Fragile X syndrome (FXS) is the most common inherited neurodevelopmental disorder with intellectual disability. Here, we show that the expression of tissue plasminogen activator (tPA) is increased in glial cells differentiated from neural progenitors of Fmr1 knock-out mice, a mouse model for FXS, and that tPA is involved in the altered migration and differentiation of these progenitors lacking FMR1 protein (FMRP). When tPA function is blocked with an antibody, enhanced migration of doublecortin-immunoreactive neurons in 1 d differentiated FMRP-deficient neurospheres is normalized. In time-lapse imaging, blocking the tPA function promotes early glial differentiation and reduces the velocity of nuclear movement of FMRP-deficient radial glia. In addition, we show that enhanced intracellular Ca(2+) responses to depolarization with potassium are prevented by the treatment with the tPA-neutralizing antibody in FMRP-deficient cells during early neural progenitor differentiation. Alterations of the tPA expression in the embryonic, postnatal, and adult brain of Fmr1 knock-out mice suggest an important role for tPA in the abnormal neuronal differentiation and plasticity in FXS. Altogether, the results indicate that tPA may prove to be an interesting potential target for pharmacological intervention in FXS.
Keywords: FMRP; depolarization; differentiation; glia; neurons; stem cells.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
