Cerebellar potentiation and learning a whisker-based object localization task with a time response window
- PMID: 24478374
- PMCID: PMC6827592
- DOI: 10.1523/JNEUROSCI.2966-13.2014
Cerebellar potentiation and learning a whisker-based object localization task with a time response window
Abstract
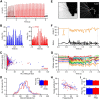
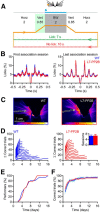
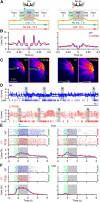
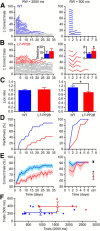
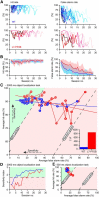
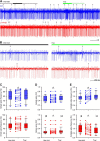
Whisker-based object localization requires activation and plasticity of somatosensory and motor cortex. These parts of the cerebral cortex receive strong projections from the cerebellum via the thalamus, but it is unclear whether and to what extent cerebellar processing may contribute to such a sensorimotor task. Here, we subjected knock-out mice, which suffer from impaired intrinsic plasticity in their Purkinje cells and long-term potentiation at their parallel fiber-to-Purkinje cell synapses (L7-PP2B), to an object localization task with a time response window (RW). Water-deprived animals had to learn to localize an object with their whiskers, and based upon this location they were trained to lick within a particular period ("go" trial) or refrain from licking ("no-go" trial). L7-PP2B mice were not ataxic and showed proper basic motor performance during whisking and licking, but were severely impaired in learning this task compared with wild-type littermates. Significantly fewer L7-PP2B mice were able to learn the task at long RWs. Those L7-PP2B mice that eventually learned the task made unstable progress, were significantly slower in learning, and showed deficiencies in temporal tuning. These differences became greater as the RW became narrower. Trained wild-type mice, but not L7-PP2B mice, showed a net increase in simple spikes and complex spikes of their Purkinje cells during the task. We conclude that cerebellar processing, and potentiation in particular, can contribute to learning a whisker-based object localization task when timing is relevant. This study points toward a relevant role of cerebellum-cerebrum interaction in a sophisticated cognitive task requiring strict temporal processing.
Figures

References
-
- Axelrad H, Crepel F. Représentation sélective des vibrisses mystaciales au niveau des cellules de Purkinje du cervelet par la voie de fibres grimpantes chez le rat. C R Acad Sci Hebd Seances Acad Sci D. 1977;284:1321–1324. - PubMed
-
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003;89:104–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
