Tie2 and Eph receptor tyrosine kinase activation and signaling
- PMID: 24478383
- PMCID: PMC3949358
- DOI: 10.1101/cshperspect.a009142
Tie2 and Eph receptor tyrosine kinase activation and signaling
Abstract
The Eph and Tie cell surface receptors mediate a variety of signaling events during development and in the adult organism. As other receptor tyrosine kinases, they are activated on binding of extracellular ligands and their catalytic activity is tightly regulated on multiple levels. The Eph and Tie receptors display some unique characteristics, including the requirement of ligand-induced receptor clustering for efficient signaling. Interestingly, both Ephs and Ties can mediate different, even opposite, biological effects depending on the specific ligand eliciting the response and on the cellular context. Here we discuss the structural features of these receptors, their interactions with various ligands, as well as functional implications for downstream signaling initiation. The Eph/ephrin structures are already well reviewed and we only provide a brief overview on the initial binding events. We go into more detail discussing the Tie-angiopoietin structures and recognition.
Figures

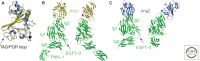

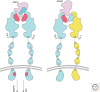

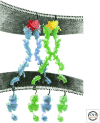
References
-
- Abengozar MA, de Frutos S, Ferreiro S, Soriano J, Perez-Martinez M, Olmeda D, Marenchino M, Canamero M, Ortega S, Megias D, et al. 2012. Blocking ephrinB2 with highly specific antibodies inhibits angiogenesis, lymphangiogenesis, and tumor growth. Blood 119: 4565–4576 - PubMed
-
- Adams RH, Alitalo K 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev 8: 464–478 - PubMed
-
- Artemenko EO, Egorova NS, Arseniev AS, Feofanov AV 2008. Transmembrane domain of EphA1 receptor forms dimers in membrane-like environment. Biochim Biophys Acta 1778: 2361–2367 - PubMed
-
- Barton WA, Tzvetkova D, Nikolov DB 2005. Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure 13: 825–832 - PubMed
-
- Barton WA, Tzvetkova-Robev D, Miranda EP, Kolev MV, Rajashankar KR, Himanen JP, Nikolov DB 2006. Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat Struct Mol Biol 13: 524–532 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
