TRIM5α and TRIM22 are differentially regulated according to HIV-1 infection phase and compartment
- PMID: 24478420
- PMCID: PMC3993776
- DOI: 10.1128/JVI.03603-13
TRIM5α and TRIM22 are differentially regulated according to HIV-1 infection phase and compartment
Abstract
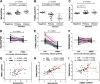
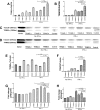
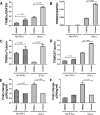
The antiviral role of TRIM E3 ligases in vivo is not fully understood. To test the hypothesis that TRIM5α and TRIM22 have differential transcriptional regulation and distinct anti-HIV roles according to infection phase and compartment, we measured TRIM5α, TRIM22, and type I interferon (IFN-I)-inducible myxovirus resistance protein A (MxA) levels in peripheral blood mononuclear cells (PBMCs) during primary and chronic HIV-1 infection, with chronic infection samples being matched PBMCs and central nervous system (CNS)-derived cells. Associations with biomarkers of disease progression were explored. The impact of IFN-I, select proinflammatory cytokines, and HIV on TRIM E3 ligase-specific expression was investigated. PBMCs from individuals with primary and chronic HIV-1 infection had significantly higher levels of MxA and TRIM22 than did PBMCs from HIV-1-negative individuals (P < 0.05 for all comparisons). PBMCs from chronic infection had lower levels of TRIM5α than did PBMCs from primary infection or HIV-1-uninfected PBMCs (P = 0.0001 for both). In matched CNS-derived samples and PBMCs, higher levels of MxA (P = 0.001) and TRIM5α (P = 0.0001) in the CNS were noted. There was a negative correlation between TRIM22 levels in PBMCs and plasma viral load (r = -0.40; P = 0.04). In vitro, IFN-I and, rarely, proinflammatory cytokines induced TRIM5α and TRIM22 in a cell type-dependent manner, and the knockdown of either protein in CD4(+) lymphocytes resulted in increased HIV-1 infection. These data suggest that there are infection-phase-specific and anatomically compartmentalized differences in TRIM5α and TRIM22 regulation involving primarily IFN-I and specific cell types and indicate subtle differences in the antiviral roles and transcriptional regulation of TRIM E3 ligases in vivo.
Importance: Type I interferon-inducible TRIM E3 ligases are a family of intracellular proteins with potent antiviral activities mediated through diverse mechanisms. However, little is known about the contribution of these proteins to antiviral immunity in vivo and how their expression is regulated. We show here that TRIM5α and TRIM22, two prominent members of the family, have different expression patterns in vivo and that the expression pattern depends on HIV-1 infection status and phase. Furthermore, expression differs in peripheral blood versus central nervous system anatomical sites of infection. Only TRIM22 expression correlated negatively with HIV-1 viral load, but gene silencing of both proteins enhances HIV-1 infection of target cells. We report subtle differences in TRIM5α and TRIM22 gene induction by IFN-I and proinflammatory cytokines in CD4(+) lymphocytes, monocytes, and neuronal cells. This study enhances our understanding of antiviral immunity by intrinsic antiviral factors and how their expression is determined.
Figures



Similar articles
-
Association of TRIM22 with the type 1 interferon response and viral control during primary HIV-1 infection.J Virol. 2011 Jan;85(1):208-16. doi: 10.1128/JVI.01810-10. Epub 2010 Oct 27. J Virol. 2011. PMID: 20980524 Free PMC article.
-
Increased expression and dysregulated association of restriction factors and type I interferon in HIV, HCV mono- and co-infected patients.J Med Virol. 2016 Jun;88(6):987-95. doi: 10.1002/jmv.24419. Epub 2015 Nov 9. J Med Virol. 2016. PMID: 26519943
-
The ELF3-TRIM22-MAVS signaling axis regulates type I interferon and antiviral responses.J Virol. 2025 May 20;99(5):e0000425. doi: 10.1128/jvi.00004-25. Epub 2025 Mar 31. J Virol. 2025. PMID: 40162781 Free PMC article.
-
TRIM22. A Multitasking Antiviral Factor.Cells. 2021 Jul 23;10(8):1864. doi: 10.3390/cells10081864. Cells. 2021. PMID: 34440633 Free PMC article. Review.
-
The interferon-stimulated gene TRIM22: A double-edged sword in HIV-1 infection.Cytokine Growth Factor Rev. 2018 Apr;40:40-47. doi: 10.1016/j.cytogfr.2018.02.001. Epub 2018 Feb 10. Cytokine Growth Factor Rev. 2018. PMID: 29650252 Review.
Cited by
-
Antigen specificities of HIV-infected cells: A role in infection and persistence?J Virus Erad. 2023 Jun 1;9(2):100329. doi: 10.1016/j.jve.2023.100329. eCollection 2023 Jun. J Virus Erad. 2023. PMID: 37440870 Free PMC article. Review.
-
DECODE: an integrated differential co-expression and differential expression analysis of gene expression data.BMC Bioinformatics. 2015 May 31;16:182. doi: 10.1186/s12859-015-0582-4. BMC Bioinformatics. 2015. PMID: 26026612 Free PMC article.
-
Genome-wide scan in two groups of HIV-infected patients treated with dendritic cell-based immunotherapy.Immunol Res. 2016 Dec;64(5-6):1207-1215. doi: 10.1007/s12026-016-8875-x. Immunol Res. 2016. PMID: 27704462
-
Nuclear localization signal in TRIM22 is essential for inhibition of type 2 porcine reproductive and respiratory syndrome virus replication in MARC-145 cells.Virus Genes. 2019 Oct;55(5):660-672. doi: 10.1007/s11262-019-01691-x. Epub 2019 Aug 2. Virus Genes. 2019. PMID: 31375995 Free PMC article.
-
On the relationship between tripartite motif-containing 22 single-nucleotide polymorphisms and COVID-19 infection severity.Hum Genomics. 2022 Aug 26;16(1):33. doi: 10.1186/s40246-022-00394-z. Hum Genomics. 2022. PMID: 36028902 Free PMC article.
References
-
- Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, Herpin B, Walker R, Deyton L, Davey RT, Jr, Falloon J, Polis MA, Salzman NP, Baseler M, Masur H, Fauci AS. 1990. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann. Intern. Med. 112:805–811 - PubMed
-
- Kovacs JA, Bechtel C, Davey RT, Jr, Falloon J, Polis MA, Walker RE, Metcalf JA, Davey V, Piscitelli SC, Baseler M, Dewar R, Salzman NP, Masur H, Lane HC. 1996. Combination therapy with didanosine and interferon-alpha in human immunodeficiency virus-infected patients: results of a phase I/II trial. J. Infect. Dis. 173:840–848. 10.1093/infdis/173.4.840 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

