Economic evaluation of laboratory testing strategies for hospital-associated Clostridium difficile infection
- PMID: 24478478
- PMCID: PMC3911327
- DOI: 10.1128/JCM.02777-13
Economic evaluation of laboratory testing strategies for hospital-associated Clostridium difficile infection
Abstract
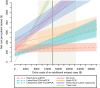
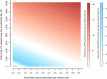
Clostridium difficile infection (CDI) is the most common cause of infectious diarrhea in health care settings, and for patients presumed to have CDI, their isolation while awaiting laboratory results is costly. Newer rapid tests for CDI may reduce this burden, but the economic consequences of different testing algorithms remain unexplored. We used decision analysis from the hospital perspective to compare multiple CDI testing algorithms for adult inpatients with suspected CDI, assuming patient management according to laboratory results. CDI testing strategies included combinations of on-demand PCR (odPCR), batch PCR, lateral-flow diagnostics, plate-reader enzyme immunoassay, and direct tissue culture cytotoxicity. In the reference scenario, algorithms incorporating rapid testing were cost-effective relative to nonrapid algorithms. For every 10,000 symptomatic adults, relative to a strategy of treating nobody, lateral-flow glutamate dehydrogenase (GDH)/odPCR generated 831 true-positive results and cost $1,600 per additional true-positive case treated. Stand-alone odPCR was more effective and more expensive, identifying 174 additional true-positive cases at $6,900 per additional case treated. All other testing strategies were dominated by (i.e., more costly and less effective than) stand-alone odPCR or odPCR preceded by lateral-flow screening. A cost-benefit analysis (including estimated costs of missed cases) favored stand-alone odPCR in most settings but favored odPCR preceded by lateral-flow testing if a missed CDI case resulted in less than $5,000 of extended hospital stay costs and <2 transmissions, if lateral-flow GDH diagnostic sensitivity was >93%, or if the symptomatic carrier proportion among the toxigenic culture-positive cases was >80%. These results can aid guideline developers and laboratory directors who are considering rapid testing algorithms for diagnosing CDI.
Figures
Similar articles
-
A cost of illness comparison for toxigenic Clostridioides difficile diagnosis algorithms in developing countries.Anaerobe. 2021 Aug;70:102390. doi: 10.1016/j.anaerobe.2021.102390. Epub 2021 May 28. Anaerobe. 2021. PMID: 34058377
-
Current knowledge on the laboratory diagnosis of Clostridium difficile infection.World J Gastroenterol. 2017 Mar 7;23(9):1552-1567. doi: 10.3748/wjg.v23.i9.1552. World J Gastroenterol. 2017. PMID: 28321156 Free PMC article. Review.
-
Clostridium difficile infection diagnosis in a paediatric population: comparison of methodologies.Eur J Clin Microbiol Infect Dis. 2014 Sep;33(9):1555-64. doi: 10.1007/s10096-014-2108-9. Epub 2014 Apr 30. Eur J Clin Microbiol Infect Dis. 2014. PMID: 24781004
-
Real-time polymerase chain reaction correlates well with clinical diagnosis of Clostridium difficile infection.J Hosp Infect. 2014 Jun;87(2):109-14. doi: 10.1016/j.jhin.2014.03.005. Epub 2014 Apr 12. J Hosp Infect. 2014. PMID: 24795170
-
How to: diagnose infection caused by Clostridium difficile.Clin Microbiol Infect. 2018 May;24(5):463-468. doi: 10.1016/j.cmi.2017.12.005. Epub 2017 Dec 18. Clin Microbiol Infect. 2018. PMID: 29269092 Review.
Cited by
-
Budget Impact Analysis of Adopting a One-Step Nucleic Acid Amplification Testing (NAAT) Alone Diagnostic Pathway for Clostridioides difficile in Japan Compared to a Two-Step Algorithm with Glutamate Dehydrogenase/Toxin Followed by NAAT.Diagnostics (Basel). 2023 Apr 18;13(8):1463. doi: 10.3390/diagnostics13081463. Diagnostics (Basel). 2023. PMID: 37189564 Free PMC article.
-
Evaluation of the Cepheid Xpert C. difficile diagnostic assay: an update meta-analysis.Braz J Microbiol. 2021 Dec;52(4):1937-1949. doi: 10.1007/s42770-021-00563-7. Epub 2021 Aug 29. Braz J Microbiol. 2021. PMID: 34455573 Free PMC article.
-
Point-Counterpoint: What Is the Optimal Approach for Detection of Clostridium difficile Infection?J Clin Microbiol. 2017 Mar;55(3):670-680. doi: 10.1128/JCM.02463-16. Epub 2017 Jan 11. J Clin Microbiol. 2017. PMID: 28077697 Free PMC article.
-
Laboratory Tests for the Diagnosis of Clostridium difficile.Clin Colon Rectal Surg. 2020 Mar;33(2):73-81. doi: 10.1055/s-0039-3400476. Epub 2020 Feb 25. Clin Colon Rectal Surg. 2020. PMID: 32104159 Free PMC article. Review.
-
Clostridium difficile outbreak caused by NAP1/BI/027 strain and non-027 strains in a Mexican hospital.Braz J Infect Dis. 2016 Jan-Feb;20(1):8-13. doi: 10.1016/j.bjid.2015.09.008. Epub 2015 Nov 24. Braz J Infect Dis. 2016. PMID: 26620948 Free PMC article.
References
-
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare Epidemiology of America, Infectious Diseases Society of America 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol. 31:431–455. 10.1086/651706 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

