Field evaluation of dried blood spots for routine HIV-1 viral load and drug resistance monitoring in patients receiving antiretroviral therapy in Africa and Asia
- PMID: 24478491
- PMCID: PMC3911301
- DOI: 10.1128/JCM.02860-13
Field evaluation of dried blood spots for routine HIV-1 viral load and drug resistance monitoring in patients receiving antiretroviral therapy in Africa and Asia
Abstract
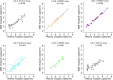
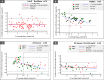
Dried blood spots (DBS) can be used in developing countries to alleviate the logistic constraints of using blood plasma specimens for viral load (VL) and HIV drug resistance (HIVDR) testing, but they should be assessed under field conditions. Between 2009 and 2011, we collected paired plasma-DBS samples from treatment-experienced HIV-1-infected adults in Burkina Faso, Cameroon, Senegal, Togo, Thailand, and Vietnam. The DBS were stored at an ambient temperature for 2 to 4 weeks and subsequently at -20°C before testing. VL testing was performed on the plasma samples and DBS using locally available methods: the Abbott m2000rt HIV-1 test, generic G2 real-time PCR, or the NucliSENS EasyQ version 1.2 test. In the case of virological failure (VF), i.e., a plasma VL of ≥1,000 copies/ml, HIVDR genotyping was performed on paired plasma-DBS samples. Overall, we compared 382 plasma-DBS sample pairs for DBS VL testing accuracy. The sensitivities of the different assays in different laboratories for detecting VF using DBS varied from 75% to 100% for the m2000rt test in labs B, C, and D, 91% to 93% for generic G2 real-time PCR in labs A and F, and 85% for the NucliSENS test in lab E. The specificities varied from 82% to 97% for the m2000rt and NucliSENS tests and reached only 60% for the generic G2 test. The NucliSENS test showed good agreement between plasma and DBS VL but underestimated the DBS VL. The lowest agreement was observed for the generic G2 test. Genotyping was successful for 96/124 (77%) DBS tested, and 75/96 (78%) plasma-DBS pairs had identical HIVDR mutations. Significant discrepancies in resistance interpretations were observed in 9 cases, 6 of which were from the same laboratory. DBS can be successfully used as an alternative to blood plasma samples for routine VL and HIVDR monitoring in African and Asian settings. However, the selection of an adequate VL measurement method and the definition of the VF threshold should be considered, and laboratory performance should be monitored.
Figures
Comment in
-
Use of reverse transcription-PCR-based assays for quantification of HIV-1 in dried blood spots requires specific HIV-1 RNA isolation for monitoring of antiretroviral treatment efficiency.J Clin Microbiol. 2014 Jul;52(7):2740-1. doi: 10.1128/JCM.00962-14. J Clin Microbiol. 2014. PMID: 24970642 Free PMC article. No abstract available.
References
-
- Aghokeng AF, Monleau M, Eymard-Duvernay S, Dagnra A, Kania D, Ngo-Giang-Huong N, Toni TD, Touré-Kane C, Truong LX, Delaporte E, Chaix ML, Peeters M, Ayouba A, ANRS 12186 Study Group 2013. Extraordinary heterogeneity of virological outcomes in patients receiving HAART and monitored with the World Health Organization (WHO) public health approach in sub-Saharan Africa and southeast Asia. Clin. Infect. Dis., in press. 10.1093/cid/cit627 - DOI - PubMed
-
- Messou E, Chaix ML, Gabillard D, Yapo V, Toni TD, Minga A, Kouakou MG, Ouattara E, Rouzioux C, Danel C, Eholie SP, Anglaret X. 2013. Increasing rate of TAMs and etravirine resistance in HIV-1-infected adults between 12 and 24 months of treatment: the VOLTART cohort study in Cote d'Ivoire, West Africa. J. Acquir. Immune Defic. Syndr. 64:211–219. 10.1097/QAI.0b013e3182a009e4 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

