A cost-effective approach for detection of toxigenic Clostridium difficile: toxigenic culture using ChromID Clostridium difficile agar
- PMID: 24478510
- PMCID: PMC3911300
- DOI: 10.1128/JCM.03113-13
A cost-effective approach for detection of toxigenic Clostridium difficile: toxigenic culture using ChromID Clostridium difficile agar
Abstract
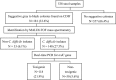
We evaluated the performance and the cost of toxigenic culture using a commercial chromogenic medium (CDIF) for 538 stool specimens. Compared with real-time PCR, this method was found to detect an additional 9% of positive specimens and result in 61% reduction in material costs, with a trade-off increase in turnaround time of 1 day.
Figures
References
-
- Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. 1978. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75:778–782 - PubMed
-
- Deshpande A, Pasupuleti V, Rolston DD, Jain A, Deshpande N, Pant C, Hernandez AV. 2011. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile infection: a metaanalysis. Clin. Infect. Dis. 53:e81–e90. 10.1093/cid/cir505 - DOI - PubMed
-
- Ferguson JK, Cheng AC, Gilbert GL, Gottlieb T, Korman T, McGregor A, Richards M, Roberts S, Robson J, Van Gessel H, Riley TV. 2011. Clostridium difficile laboratory testing in Australia and New Zealand: national survey results and Australasian Society for Infectious Diseases recommendations for best practice. Pathology 43:482–487. 10.1097/PAT.0b013e328348c9b4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources