The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties
- PMID: 24478634
- PMCID: PMC3904115
- DOI: 10.3389/fncir.2013.00096
The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties
Abstract
The reflexological view of brain function (Sherrington, 1906) has played a crucial role in defining both the nature of connectivity and the role of the synaptic interactions among neuronal circuits. One implicit assumption of this view, however, has been that CNS function is fundamentally driven by sensory input. This view was questioned as early as the beginning of the last century when a possible role for intrinsic activity in CNS function was proposed by Thomas Graham Brow (Brown, 1911, 1914). However, little progress was made in addressing intrinsic neuronal properties in vertebrates until the discovery of calcium conductances in vertebrate central neurons leading dendritic electroresponsiveness (Llinás and Hess, 1976; Llinás and Sugimori, 1980a,b) and subthreshold neuronal oscillation in mammalian inferior olive (IO) neurons (Llinás and Yarom, 1981a,b). This happened in parallel with a similar set of findings concerning invertebrate neuronal system (Marder and Bucher, 2001). The generalization into a more global view of intrinsic rhythmicity, at forebrain level, occurred initially with the demonstration that the thalamus has similar oscillatory properties (Llinás and Jahnsen, 1982) and the ionic properties responsible for some oscillatory activity were, in fact, similar to those in the IO (Jahnsen and Llinás, 1984; Llinás, 1988). Thus, lending support to the view that not only motricity, but cognitive properties, are organized as coherent oscillatory states (Pare et al., 1992; Singer, 1993; Hardcastle, 1997; Llinás et al., 1998; Varela et al., 2001).
Keywords: IO neurons; PO neuron oscillation; electrophysiology; intrinsic oscillatory; olivo-cerebellar.
Figures



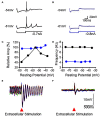
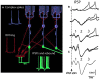
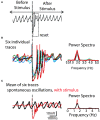
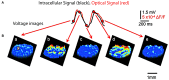
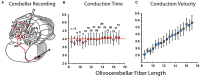
References
-
- Ariens-Kappers C. U., Huber G. C., Crosby E. C. (1936). The Comparative Anatomy of the Nervous System of Vertebrates Including Man. New York, NY: Macmillan
-
- Bal T., McCormick D. A. (1997). Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h). J. Neurophysiol. 77, 3145–3156 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

