Sleep and protein synthesis-dependent synaptic plasticity: impacts of sleep loss and stress
- PMID: 24478645
- PMCID: PMC3896837
- DOI: 10.3389/fnbeh.2013.00224
Sleep and protein synthesis-dependent synaptic plasticity: impacts of sleep loss and stress
Abstract
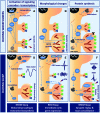
Sleep has been ascribed a critical role in cognitive functioning. Several lines of evidence implicate sleep in the consolidation of synaptic plasticity and long-term memory. Stress disrupts sleep while impairing synaptic plasticity and cognitive performance. Here, we discuss evidence linking sleep to mechanisms of protein synthesis-dependent synaptic plasticity and synaptic scaling. We then consider how disruption of sleep by acute and chronic stress may impair these mechanisms and degrade sleep function.
Keywords: Arc/Arg3.1; brain-derived neurotrophic factor; gene expression; long-term potentiation; mood disorder; sleep deprivation; stress; translation control.
Figures
References
-
- Artola A., von Frijtag J. C., Fermont P. C., Gispen W. H., Schrama L. H., Kamal A., et al. (2006). Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur. J. Neurosci. 23, 261–272 10.1111/j.1460-9568.2005.04552.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

