The neuronal and molecular basis of quinine-dependent bitter taste signaling in Drosophila larvae
- PMID: 24478653
- PMCID: PMC3902218
- DOI: 10.3389/fnbeh.2014.00006
The neuronal and molecular basis of quinine-dependent bitter taste signaling in Drosophila larvae
Abstract
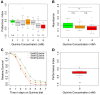
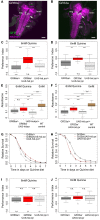
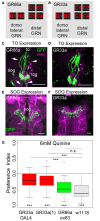
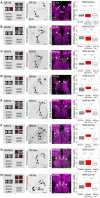
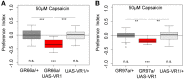
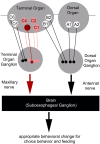
The sensation of bitter substances can alert an animal that a specific type of food is harmful and should not be consumed. However, not all bitter compounds are equally toxic and some may even be beneficial in certain contexts. Thus, taste systems in general may have a broader range of functions than just in alerting the animal. In this study we investigate bitter sensing and processing in Drosophila larvae using quinine, a substance perceived by humans as bitter. We show that behavioral choice, feeding, survival, and associative olfactory learning are all directly affected by quinine. On the cellular level, we show that 12 gustatory sensory receptor neurons that express both GR66a and GR33a are required for quinine-dependent choice and feeding behavior. Interestingly, these neurons are not necessary for quinine-dependent survival or associative learning. On the molecular receptor gene level, the GR33a receptor, but not GR66a, is required for quinine-dependent choice behavior. A screen for gustatory sensory receptor neurons that trigger quinine-dependent choice behavior revealed that a single GR97a receptor gene expressing neuron located in the peripheral terminal sense organ is partially necessary and sufficient. For the first time, we show that the elementary chemosensory system of the Drosophila larva can serve as a simple model to understand the neuronal basis of taste information processing on the single cell level with respect to different behavioral outputs.
Keywords: Drosophila larvae; bitter; feeding; gustation; gustatory receptors; learning and memory; single cell.
Figures
References
-
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

