Role of AE2 for pHi regulation in biliary epithelial cells
- PMID: 24478713
- PMCID: PMC3894451
- DOI: 10.3389/fphys.2013.00413
Role of AE2 for pHi regulation in biliary epithelial cells
Abstract
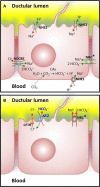
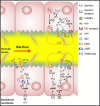
The Cl(-)/HCO(-) 3anion exchanger 2 (AE2) is known to be involved in intracellular pH (pHi) regulation and transepithelial acid-base transport. Early studies showed that AE2 gene expression is reduced in liver biopsies and blood mononuclear cells from patients with primary biliary cirrhosis (PBC), a disease characterized by chronic non-suppurative cholangitis associated with antimitochondrial antibodies (AMA) and other autoimmune phenomena. Microfluorimetric analysis of the Cl(-)/HCO(-) 3 anion exchange (AE) in isolated cholangiocytes showed that the cAMP-stimulated AE activity is diminished in PBC compared to both healthy and diseased controls. More recently, it was found that miR-506 is upregulated in cholangiocytes of PBC patients and that AE2 may be a target of miR-506. Additional evidence for a pathogenic role of AE2 dysregulation in PBC was obtained with Ae2 (-/-) a,b mice, which develop biochemical, histological, and immunologic alterations that resemble PBC (including development of serum AMA). Analysis of HCO(-) 3 transport systems and pHi regulation in cholangiocytes from normal and Ae2 (-/-) a,b mice confirmed that AE2 is the transporter responsible for the Cl(-)/HCO(-) 3exchange in these cells. On the other hand, both Ae2 (+/+) a,b and Ae2 (-/-) a,b mouse cholangiocytes exhibited a Cl(-)-independent bicarbonate transport system, essentially a Na(+)-bicarbonate cotransport (NBC) system, which could contribute to pHi regulation in the absence of AE2.
Keywords: Cl−/HCO−3 anion exchange; bile flow; biliary HCO−3 secretion; cholangiocytes; primary biliary cirrhosis.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

