Central and direct regulation of testicular activity by gonadotropin-inhibitory hormone and its receptor
- PMID: 24478760
- PMCID: PMC3902780
- DOI: 10.3389/fendo.2014.00008
Central and direct regulation of testicular activity by gonadotropin-inhibitory hormone and its receptor
Abstract
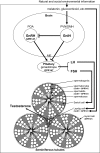
Gonadotropin-inhibitory hormone (GnIH) was first identified in Japanese quail to be an inhibitor of gonadotropin synthesis and release. GnIH peptides have since been identified in all vertebrates, and all share an LPXRFamide (X = L or Q) motif at their C-termini. The receptor for GnIH is the G protein-coupled receptor 147 (GPR147), which inhibits cAMP signaling. Cell bodies of GnIH neurons are located in the paraventricular nucleus (PVN) in birds and the dorsomedial hypothalamic area (DMH) in most mammals. GnIH neurons in the PVN or DMH project to the median eminence to control anterior pituitary function via GPR147 expressed in gonadotropes. Further, GnIH inhibits gonadotropin-releasing hormone (GnRH)-induced gonadotropin subunit gene transcription by inhibiting the adenylate cyclase/cAMP/PKA-dependent ERK pathway in an immortalized mouse gonadotrope cell line (LβT2 cells). GnIH neurons also project to GnRH neurons that express GPR147 in the preoptic area (POA) in birds and mammals. Accordingly, GnIH can inhibit gonadotropin synthesis and release by decreasing the activity of GnRH neurons as well as by directly inhibiting pituitary gonadotrope activity. GnIH and GPR147 can thus centrally suppress testosterone secretion and spermatogenesis by acting in the hypothalamic-pituitary-gonadal axis. GnIH and GPR147 are also expressed in the testis of birds and mammals, possibly acting in an autocrine/paracrine manner to suppress testosterone secretion and spermatogenesis. GnIH expression is also regulated by melatonin, stress, and social environment in birds and mammals. Accordingly, the GnIH-GPR147 system may play a role in transducing physical and social environmental information to regulate optimal testicular activity in birds and mammals. This review discusses central and direct inhibitory effects of GnIH and GPR147 on testosterone secretion and spermatogenesis in birds and mammals.
Keywords: GPR147; gonadotropin-inhibitory hormone; gonadotropins; melatonin; social environment; spermatogenesis; stress; testosterone.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

