Localized electron transfer rates and microelectrode-based enrichment of microbial communities within a phototrophic microbial mat
- PMID: 24478768
- PMCID: PMC3902354
- DOI: 10.3389/fmicb.2014.00011
Localized electron transfer rates and microelectrode-based enrichment of microbial communities within a phototrophic microbial mat
Abstract
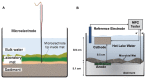
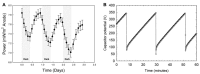
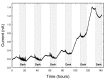
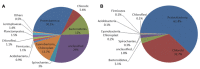
Phototrophic microbial mats frequently exhibit sharp, light-dependent redox gradients that regulate microbial respiration on specific electron acceptors as a function of depth. In this work, a benthic phototrophic microbial mat from Hot Lake, a hypersaline, epsomitic lake located near Oroville in north-central Washington, was used to develop a microscale electrochemical method to study local electron transfer processes within the mat. To characterize the physicochemical variables influencing electron transfer, we initially quantified redox potential, pH, and dissolved oxygen gradients by depth in the mat under photic and aphotic conditions. We further demonstrated that power output of a mat fuel cell was light-dependent. To study local electron transfer processes, we deployed a microscale electrode (microelectrode) with tip size ~20 μm. To enrich a subset of microorganisms capable of interacting with the microelectrode, we anodically polarized the microelectrode at depth in the mat. Subsequently, to characterize the microelectrode-associated community and compare it to the neighboring mat community, we performed amplicon sequencing of the V1-V3 region of the 16S gene. Differences in Bray-Curtis beta diversity, illustrated by large changes in relative abundance at the phylum level, suggested successful enrichment of specific mat community members on the microelectrode surface. The microelectrode-associated community exhibited substantially reduced alpha diversity and elevated relative abundances of Prosthecochloris, Loktanella, Catellibacterium, other unclassified members of Rhodobacteraceae, Thiomicrospira, and Limnobacter, compared with the community at an equivalent depth in the mat. Our results suggest that local electron transfer to an anodically polarized microelectrode selected for a specific microbial population, with substantially more abundance and diversity of sulfur-oxidizing phylotypes compared with the neighboring mat community.
Keywords: electron transfer; hot lake; microbial mats; microelectrodes; sequence analysis; sulfur cycle.
Figures




References
-
- Anderson G. C. (1958). Some limnological features of a shallow saline meromictic lake. Limnol. Oceanogr. 3 259–270 10.4319/lo.1958.3.3.0259 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

