Human immunodeficiency syndromes affecting human natural killer cell cytolytic activity
- PMID: 24478771
- PMCID: PMC3896857
- DOI: 10.3389/fimmu.2014.00002
Human immunodeficiency syndromes affecting human natural killer cell cytolytic activity
Abstract
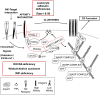
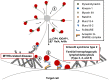
Natural killer (NK) cells are lymphocytes of the innate immune system that secrete cytokines upon activation and mediate the killing of tumor cells and virus-infected cells, especially those that escape the adaptive T cell response caused by the down regulation of MHC-I. The induction of cytotoxicity requires that NK cells contact target cells through adhesion receptors, and initiate activation signaling leading to increased adhesion and accumulation of F-actin at the NK cell cytotoxic synapse. Concurrently, lytic granules undergo minus-end directed movement and accumulate at the microtubule-organizing center through the interaction with microtubule motor proteins, followed by polarization of the lethal cargo toward the target cell. Ultimately, myosin-dependent movement of the lytic granules toward the NK cell plasma membrane through F-actin channels, along with soluble N-ethylmaleimide-sensitive factor attachment protein receptor-dependent fusion, promotes the release of the lytic granule contents into the cleft between the NK cell and target cell resulting in target cell killing. Herein, we will discuss several disease-causing mutations in primary immunodeficiency syndromes and how they impact NK cell-mediated killing by disrupting distinct steps of this tightly regulated process.
Keywords: NK cell cytotoxicity; NK cells; cytotoxic lymphocytes; cytotoxic synapse; lytic granules; primary immunodeficiency.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

