When fat is not bad: the regulation of actin dynamics by phospholipid signaling molecules
- PMID: 24478785
- PMCID: PMC3899574
- DOI: 10.3389/fpls.2014.00005
When fat is not bad: the regulation of actin dynamics by phospholipid signaling molecules
Abstract
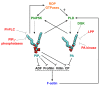
The actin cytoskeleton plays a key role in the plant morphogenesis and is involved in polar cell growth, movement of subcellular organelles, cell division, and plant defense. Organization of actin cytoskeleton undergoes dynamic remodeling in response to internal developmental cues and diverse environmental signals. This dynamic behavior is regulated by numerous actin-binding proteins (ABPs) that integrate various signaling pathways. Production of the signaling lipids phosphatidylinositol 4,5-bisphosphate and phosphatidic acid affects the activity and subcellular distribution of several ABPs, and typically correlates with increased actin polymerization. Here we review current knowledge of the inter-regulatory dynamics between signaling phospholipids and the actin cytoskeleton in plant cells.
Keywords: actin; actin-binding proteins; capping protein; cytoskeleton; phosphatidic acid; phosphatidylinositol 4,5-bisphosphate; phospholipase D; signaling.
Figures
Similar articles
-
Structural insights into the inhibition of actin-capping protein by interactions with phosphatidic acid and phosphatidylinositol (4,5)-bisphosphate.PLoS Comput Biol. 2012;8(11):e1002765. doi: 10.1371/journal.pcbi.1002765. Epub 2012 Nov 1. PLoS Comput Biol. 2012. PMID: 23133367 Free PMC article.
-
Arabidopsis capping protein senses cellular phosphatidic acid levels and transduces these into changes in actin cytoskeleton dynamics.Plant Signal Behav. 2012 Dec;7(12):1727-30. doi: 10.4161/psb.22472. Epub 2012 Oct 16. Plant Signal Behav. 2012. PMID: 23072985 Free PMC article.
-
Regulation of cytoskeleton-associated protein activities: Linking cellular signals to plant cytoskeletal function.J Integr Plant Biol. 2021 Jan;63(1):241-250. doi: 10.1111/jipb.13046. J Integr Plant Biol. 2021. PMID: 33274838 Review.
-
Regulation of cytoskeletal dynamics by phospholipase D and phosphatidic acid.Trends Plant Sci. 2013 Sep;18(9):496-504. doi: 10.1016/j.tplants.2013.04.005. Epub 2013 May 7. Trends Plant Sci. 2013. PMID: 23664415 Review.
-
The actin cytoskeleton and signaling network during pollen tube tip growth.J Integr Plant Biol. 2010 Feb;52(2):131-7. doi: 10.1111/j.1744-7909.2010.00922.x. J Integr Plant Biol. 2010. PMID: 20377675 Review.
Cited by
-
Phospholipase D affects translocation of NPR1 to the nucleus in Arabidopsis thaliana.Front Plant Sci. 2015 Feb 18;6:59. doi: 10.3389/fpls.2015.00059. eCollection 2015. Front Plant Sci. 2015. PMID: 25741350 Free PMC article.
-
Mechanical stress effects on transcriptional regulation of genes encoding microtubule- and actin-associated proteins.Physiol Mol Biol Plants. 2022 Jan;28(1):17-30. doi: 10.1007/s12298-021-01123-x. Epub 2022 Jan 21. Physiol Mol Biol Plants. 2022. PMID: 35210715 Free PMC article.
-
Plasma membrane phospholipid signature recruits the plant exocyst complex via the EXO70A1 subunit.Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2105287118. doi: 10.1073/pnas.2105287118. Proc Natl Acad Sci U S A. 2021. PMID: 34470819 Free PMC article.
-
A dynamic interplay between membranes and the cytoskeleton critical for cell development and signaling.Front Plant Sci. 2014 Jul 16;5:335. doi: 10.3389/fpls.2014.00335. eCollection 2014. Front Plant Sci. 2014. PMID: 25076954 Free PMC article. No abstract available.
-
Overlapping Yet Response-Specific Transcriptome Alterations Characterize the Nature of Tobacco-Pseudomonas syringae Interactions.Front Plant Sci. 2016 Mar 7;7:251. doi: 10.3389/fpls.2016.00251. eCollection 2016. Front Plant Sci. 2016. PMID: 27014286 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

