Autophagy as a possible mechanism for micronutrient remobilization from leaves to seeds
- PMID: 24478789
- PMCID: PMC3900762
- DOI: 10.3389/fpls.2014.00011
Autophagy as a possible mechanism for micronutrient remobilization from leaves to seeds
Abstract
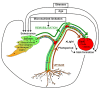
Seed formation is an important step of plant development which depends on nutrient allocation. Uptake from soil is an obvious source of nutrients which mainly occurs during vegetative stage. Because seed filling and leaf senescence are synchronized, subsequent mobilization of nutrients from vegetative organs also play an essential role in nutrient use efficiency, providing source-sink relationships. However, nutrient accumulation during the formation of seeds may be limited by their availability in source tissues. While several mechanisms contributing to make leaf macronutrients available were already described, little is known regarding micronutrients such as metals. Autophagy, which is involved in nutrient recycling, was already shown to play a critical role in nitrogen remobilization to seeds during leaf senescence. Because it is a non-specific mechanism, it could also control remobilization of metals. This article reviews actors and processes involved in metal remobilization with emphasis on autophagy and methodology to study metal fluxes inside the plant. A better understanding of metal remobilization is needed to improve metal use efficiency in the context of biofortification.
Keywords: Fe; Zn; atg; isotopic labeling; leaf senescence; nutrient fluxes; nutrient use efficiency; transition metal.
Figures

References
-
- Aggarwal A., Sharma I., Tripathi B. N., Munjal A. K., Baunthiyal M., Sharma V. (2012). “Metal toxicity and photosynthesis,” in Photosynthesis: Overviews on Recent Progress and Future Perspectives edsItoh S., Mohanty P., Guruprasad K. N. (New Delhi:IK International Publishing House (Pvt) Limited; ).
-
- Badger M. R., Price G. D. (1994). The role of carbonic anhydrase in photosynthesis. Annu. Rev. Plant Biol. 45 369–39210.1146/annurev.pp.45.060194.002101 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

