Implications of sex-specific selection for the genetic basis of disease
- PMID: 24478802
- PMCID: PMC3901550
- DOI: 10.1111/eva.12097
Implications of sex-specific selection for the genetic basis of disease
Abstract
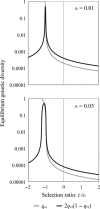
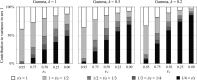
Mutation and selection are thought to shape the underlying genetic basis of many common human diseases. However, both processes depend on the context in which they occur, such as environment, genetic background, or sex. Sex has widely known effects on phenotypic expression of genotype, but an analysis of how it influences the evolutionary dynamics of disease-causing variants has not yet been explored. We develop a simple population genetic model of disease susceptibility and evaluate it using a biologically plausible empirically based distribution of fitness effects among contributing mutations. The model predicts that alleles under sex-differential selection, including sexually antagonistic alleles, will disproportionately contribute to genetic variation for disease predisposition, thereby generating substantial sexual dimorphism in the genetic architecture of complex (polygenic) diseases. This is because such alleles evolve into higher population frequencies for a given effect size, relative to alleles experiencing equally strong purifying selection in both sexes. Our results provide a theoretical justification for expecting a sexually dimorphic genetic basis for variation in complex traits such as disease. Moreover, they suggest that such dimorphism is interesting - not merely something to control for - because it reflects the action of natural selection in molding the evolution of common disease phenotypes.
Keywords: contemporary evolution; ecological genetics; evolutionary medicine; evolutionary theory; sexual selection.
Figures
Similar articles
-
Accumulation of Deleterious Mutations Near Sexually Antagonistic Genes.G3 (Bethesda). 2016 Aug 9;6(8):2273-84. doi: 10.1534/g3.116.031161. G3 (Bethesda). 2016. PMID: 27226163 Free PMC article.
-
Sex-specific natural selection on SNPs in Silene latifolia.Evol Lett. 2022 May 27;6(4):308-318. doi: 10.1002/evl3.283. eCollection 2022 Aug. Evol Lett. 2022. PMID: 35937470 Free PMC article.
-
Evolutionary Consequences of Sex-Specific Selection in Variable Environments: Four Simple Models Reveal Diverse Evolutionary Outcomes.Am Nat. 2019 Jan;193(1):93-105. doi: 10.1086/700720. Epub 2018 Nov 15. Am Nat. 2019. PMID: 30624102
-
Sexual dimorphism in flowering plants.J Exp Bot. 2013 Jan;64(1):67-82. doi: 10.1093/jxb/ers308. Epub 2012 Nov 25. J Exp Bot. 2013. PMID: 23183260 Review.
-
Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best?Behav Brain Sci. 2006 Aug;29(4):385-404; discussion 405-52. doi: 10.1017/S0140525X06009095. Behav Brain Sci. 2006. PMID: 17094843 Review.
Cited by
-
Autosomal and X-Linked Additive Genetic Variation for Lifespan and Aging: Comparisons Within and Between the Sexes in Drosophila melanogaster.G3 (Bethesda). 2016 Dec 7;6(12):3903-3911. doi: 10.1534/g3.116.028308. G3 (Bethesda). 2016. PMID: 27678519 Free PMC article.
-
Accounting for eXentricities: analysis of the X chromosome in GWAS reveals X-linked genes implicated in autoimmune diseases.PLoS One. 2014 Dec 5;9(12):e113684. doi: 10.1371/journal.pone.0113684. eCollection 2014. PLoS One. 2014. PMID: 25479423 Free PMC article.
-
The distribution of fitness effects in an uncertain world.Evolution. 2015 Jun;69(6):1610-1618. doi: 10.1111/evo.12673. Epub 2015 May 19. Evolution. 2015. PMID: 25913128 Free PMC article.
-
Sex differences in allometry for phenotypic traits in mice indicate that females are not scaled males.Nat Commun. 2022 Dec 12;13(1):7502. doi: 10.1038/s41467-022-35266-6. Nat Commun. 2022. PMID: 36509767 Free PMC article.
-
Sex Differences in Blood HDL-c, the Total Cholesterol/HDL-c Ratio, and Palmitoleic Acid are Not Associated with Variants in Common Candidate Genes.Lipids. 2017 Dec;52(12):969-980. doi: 10.1007/s11745-017-4307-5. Epub 2017 Oct 27. Lipids. 2017. PMID: 29080057 Free PMC article.
References
-
- Åslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW. Maltreatment, MAOA, and delinquency: sex differences in gene–environment interaction in a large population-based cohort of adolescents. Behavior Genetics. 2011;41:262–272. - PubMed
-
- Behrens G, Winkler TW, Gorski M, Leitzmann MF, Heid IM. To stratify or not to stratify: power considerations for population-based genome-wide association studies of quantitative traits. Genetic Epidemiology. 2011;35:867–879. - PubMed
-
- Bonduriansky R, Chenoweth SF. Intralocus sexual conflict. Trends in Ecology & Evolution. 2009;24:280–288. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

