Dkk1 in the peri-cloaca mesenchyme regulates formation of anorectal and genitourinary tracts
- PMID: 24479159
- PMCID: PMC3934837
- DOI: 10.1016/j.ydbio.2013.10.016
Dkk1 in the peri-cloaca mesenchyme regulates formation of anorectal and genitourinary tracts
Abstract
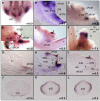
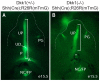
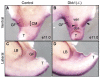
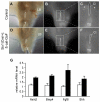
Anorectal malformation (ARM) is a common birth defect but the developmental history and the underlying molecular mechanism are poorly understood. Using murine genetic models, we report here that a signaling molecule Dickkopf-1 (Dkk1) is a critical regulator. The anorectal and genitourinary tracts are major derivatives of caudal hindgut, or the cloaca.Dkk1 is highly expressed in the dorsal peri-cloacal mesenchymal (dPCM) progenitors. We show that the deletion of Dkk1 causes the imperforate anus with rectourinary fistula. Mutant genital tubercles exhibit a preputial hypospadias phenotype and premature urethral canalization.Dkk1 mutants have an ectopic expansion of the dPCM tissue, which correlates with an aberrant increase of cell proliferation and survival. This ectopic tissue is detectable before the earliest sign of the anus formation, suggesting that it is most likely the primary or early cause of the defect. Deletion of Dkk1 results in an elevation of the Wnt/ß-catenin activity. Signaling molecules Shh, Fgf8 and Bmp4 are also upregulated. Furthermore, genetic hyperactivation of Wnt/ß-catenin signal pathway in the cloacal mesenchyme partially recapitulates Dkk1 mutant phenotypes. Together, these findings underscore the importance ofDKK1 in regulating behavior of dPCM progenitors, and suggest that formation of anus and urethral depends on Dkk1-mediated dynamic inhibition of the canonical Wnt/ß-catenin signal pathway.
© 2013 Published by Elsevier Inc.
Figures





References
-
- Cuschieri A. Anorectal anomalies associated with or as part of other anomalies. Am J Med Genet. 2002;110:122–130. - PubMed
-
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

