Varicella zoster-associated retinal and central nervous system vasculitis in a patient with multiple sclerosis treated with natalizumab
- PMID: 24479415
- PMCID: PMC3910236
- DOI: 10.1186/1742-2094-11-19
Varicella zoster-associated retinal and central nervous system vasculitis in a patient with multiple sclerosis treated with natalizumab
Abstract
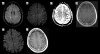
We report the first case of combined retinal and CNS varicella zoster-associated vasculitis in a 49-year-old patient with multiple sclerosis who had been treated with natalizumab. He presented with a progressive bilateral visual loss. The diagnosis of a vasculitis was based on the fundoscopic examination and MRI findings. We confirmed the varicella zoster virus (VZV) infection of the CNS by PCR and increased intrathecal antibody indices in the cerebrospinal fluid. The patient was stabilized with antiviral treatment, methylprednisolone, plasmapheresis and cycophosphamide. Natalizumab was discontinued. This case illustrates the neuroimmunological and neuroinfectiological consequences of treatments with biologicals that influence the immune system.
Figures

References
-
- Chan PY, Aruffo A. VLA-4 integrin mediates lymphocyte migration on the inducible endothelial cell ligand VCAM-1 and the extracellular matrix ligand fibronectin. J Biol Chem. 1993;268:24655–24664. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

