Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis
- PMID: 24479481
- PMCID: PMC3978827
- DOI: 10.1186/ar4467
Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis
Abstract
Introduction: Visfatin is an adipokine that may be involved in intertissular joint communication in osteoarthritis (OA). With a homodimeric conformation, it exerts nicotinamide phosphoribosyltransferase (Nampt) enzymatic activity, essential for nicotinamide adenine dinucleotide biosynthesis. We examined the tissular origin and conformation of visfatin/Nampt in human OA joints and investigated the role of visfatin/Nampt in chondrocytes and osteoblasts by studying Nampt enzymatic activity.
Methods: Synovium, cartilage and subchondral bone from human OA joints were used for protein extraction or incubated for 24 hours in serum-free media (conditioned media), and synovial fluid was obtained from OA patients. Visfatin/Nampt expression in tissular extracts and conditioned media was evaluated by western blot and enzyme-linked immunosorbent assay (ELISA), respectively. Nampt activity was assessed in OA synovium by colorimetric assay. Primary cultures of murine chondrocytes and osteoblasts were stimulated with visfatin/Nampt and pretreated or not with APO866, a pharmacologic inhibitor of Nampt activity. The effect on cytokines, chemokines, growth factors and hypertrophic markers expression was examined by quantitative reverse transcriptase polymerase chain reaction and/or ELISA.
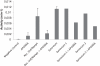
Results: In tissular explants, conditioned media and synovial fluid, visfatin/Nampt was found as a homodimer, corresponding to the enzymatically active conformation. All human OA joint tissues released visfatin/Nampt (synovium: 628 ± 106 ng/g tissue; subchondral bone: 195 ± 26 ng/g tissue; cartilage: 152 ± 46 ng/g tissue), with significantly higher level for synovium (P <0.0005). Nampt activity was identified ex vivo in synovium. In vitro, visfatin/Nampt significantly induced the expression of interleukin 6, keratinocyte chemoattractant and monocyte chemoattractant protein 1 in chondrocytes and osteoblasts. APO866 decreased the mRNA and protein levels of these pro-inflammatory cytokines in the two cell types (up to 94% and 63% inhibition, respectively). Levels of growth factors (vascular endothelial growth factor, transforming growth factor β) and hypertrophic genes were unchanged with treatment.
Conclusion: Visfatin/Nampt is released by all human OA tissues in a dimeric enzymatically active conformation and mostly by the synovium, which displays Nampt activity. The Nampt activity of visfatin is involved in chondrocyte and osteoblast activation, so targeting this enzymatic activity to disrupt joint tissue interactions may be novel in OA therapy.
Figures





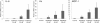

References
-
- Mahjoub M, Berenbaum F, Houard X. Why subchondral bone in osteoarthritis? The importance of the cartilage bone interface in osteoarthritis. Osteoporos Int. 2012;16:S841–S846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

