Identification of nitrated immunoglobulin variable regions in the HIV-infected human brain: implications in HIV infection and immune response
- PMID: 24479669
- PMCID: PMC4088966
- DOI: 10.1021/pr401117m
Identification of nitrated immunoglobulin variable regions in the HIV-infected human brain: implications in HIV infection and immune response
Abstract
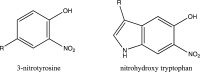
HIV can infiltrate the brain and lead to HIV-associated neurocognitive disorders (HAND). The pathophysiology of HAND is poorly understood, and there are no diagnostic biomarkers for it. Previously, an increase in inducible nitric oxide synthase levels and protein tyrosine nitration in the brain were found to correlate with the severity of HAND.1,2 In this study, we analyzed human brains from individuals who had HIV infection without encephalitis and with encephalitis/HAND and compared them to the brains of healthy individuals. We identified the nitrated proteins and determined the sites of modification using affinity enrichment followed by high-resolution and high-mass-accuracy nanoLC-MS/MS. We found that nitrated proteins were predominantly present in the HIV-infected individuals with encephalitis, and, interestingly, the modifications were predominantly located on immunoglobulin variable regions. Our molecular model indicated potential interactions with HIV envelope proteins and changes on the heavy and light chain interface upon the nitration and nitrohydroxylation of these residues. Therefore, our findings suggest a role for these modifications in the immune response, which may have implications in disease pathogenesis.
Figures



Similar articles
-
An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis.J Neurovirol. 1997 Dec;3(6):401-16. doi: 10.3109/13550289709031186. J Neurovirol. 1997. PMID: 9475112
-
HIV-1-infection in the CNS. A pathogenesis of some neurological syndromes in the light of recent investigations.Folia Neuropathol. 1998;36(4):211-6. Folia Neuropathol. 1998. PMID: 10079602 Review.
-
Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis.Am J Pathol. 2006 Apr;168(4):1335-44. doi: 10.2353/ajpath.2006.051181. Am J Pathol. 2006. PMID: 16565506 Free PMC article.
-
Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis.J Infect Dis. 2011 Jun 1;203(11):1647-57. doi: 10.1093/infdis/jir163. J Infect Dis. 2011. PMID: 21592995 Free PMC article.
-
HIV and drug misuse in the Edinburgh cohort.J Acquir Immune Defic Syndr. 2002 Oct 1;31 Suppl 2:S35-42. doi: 10.1097/00126334-200210012-00003. J Acquir Immune Defic Syndr. 2002. PMID: 12394781 Review.
Cited by
-
The role of oxidative stress in HIV-associated neurocognitive disorders.Brain Behav Immun Health. 2021 Feb 28;13:100235. doi: 10.1016/j.bbih.2021.100235. eCollection 2021 May. Brain Behav Immun Health. 2021. PMID: 34589750 Free PMC article. Review.
-
A large scale Plasmodium vivax- Saimiri boliviensis trophozoite-schizont transition proteome.PLoS One. 2017 Aug 22;12(8):e0182561. doi: 10.1371/journal.pone.0182561. eCollection 2017. PLoS One. 2017. PMID: 28829774 Free PMC article.
-
Plasmodium vivax trophozoite-stage proteomes.J Proteomics. 2015 Feb 6;115:157-76. doi: 10.1016/j.jprot.2014.12.010. Epub 2014 Dec 27. J Proteomics. 2015. PMID: 25545414 Free PMC article.
-
Mass spectrometric phosphoproteome analysis of HIV-infected brain reveals novel phosphorylation sites and differential phosphorylation patterns.Proteomics Clin Appl. 2016 Feb;10(2):126-35. doi: 10.1002/prca.201400134. Epub 2015 Aug 12. Proteomics Clin Appl. 2016. PMID: 26033855 Free PMC article.
-
Oxidative Stress during HIV Infection: Mechanisms and Consequences.Oxid Med Cell Longev. 2016;2016:8910396. doi: 10.1155/2016/8910396. Epub 2016 Oct 13. Oxid Med Cell Longev. 2016. PMID: 27829986 Free PMC article. Review.
References
-
- Boven L. A.; Gomes L.; Hery C.; Gray F.; Verhoef J.; Portegies P.; Tardieu M.; Nottet H. S. J. Immunol. 1999, 7, 4319–4327. - PubMed
-
- Castagna A.; Le Grazie C.; Accordini A.; Giulidori P.; Cavalli G.; Bottiglieri T.; Lazzarin A. Neurology 1995, 9, 1678–1683. - PubMed
-
- Choi J.; Liu R. M.; Kundu R. K.; Sangiorgi F.; Wu W.; Maxson R.; Forman H. J. J. Biol. Chem. 2000, 5, 3693–3698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

