Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies
- PMID: 24479894
- PMCID: PMC3922636
- DOI: 10.1186/2051-5960-2-14
Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies
Abstract
Background: In sporadic Tauopathies, neurofibrillary degeneration (NFD) is characterised by the intraneuronal aggregation of wild-type Tau proteins. In the human brain, the hierarchical pathways of this neurodegeneration have been well established in Alzheimer's disease (AD) and other sporadic tauopathies such as argyrophilic grain disorder and progressive supranuclear palsy but the molecular and cellular mechanisms supporting this progression are yet not known. These pathways appear to be associated with the intercellular transmission of pathology, as recently suggested in Tau transgenic mice. However, these conclusions remain ill-defined due to a lack of toxicity data and difficulties associated with the use of mutant Tau.
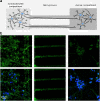
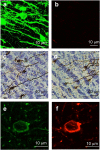
Results: Using a lentiviral-mediated rat model of hippocampal NFD, we demonstrated that wild-type human Tau protein is axonally transferred from ventral hippocampus neurons to connected secondary neurons even at distant brain areas such as olfactory and limbic systems indicating a trans-synaptic protein transfer. Using different immunological tools to follow phospho-Tau species, it was clear that Tau pathology generated using mutated Tau remains near the IS whereas it spreads much further using the wild-type one.
Conclusion: Taken together, these results support a novel mechanism for Tau protein transfer compared to previous reports based on transgenic models with mutant cDNA. It also demonstrates that mutant Tau proteins are not suitable for the development of experimental models helpful to validate therapeutic intervention interfering with Tau spreading.
Figures




References
-
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;2:95–130. - PubMed
-
- Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;2:532–544. - PubMed
-
- Chaunu MP, Deramecourt V, Buée-Scherrer V, Le Ber I, Brice A, Ehrle N, Hachimi KE, Pluot M, Maurage CA, Bakchine S, Buée L. Juvenile frontotemporal dementia with parkinsonism associated with Tau mutation G389R. J Alzheimer dis. 2013;2:769–776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

