Glucocorticoid induced leucine zipper inhibits apoptosis of cardiomyocytes by doxorubicin
- PMID: 24480152
- PMCID: PMC4131675
- DOI: 10.1016/j.taap.2014.01.013
Glucocorticoid induced leucine zipper inhibits apoptosis of cardiomyocytes by doxorubicin
Abstract
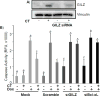
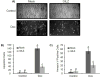
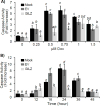
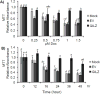
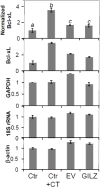
Doxorubicin (Dox) is an indispensable chemotherapeutic agent for the treatment of various forms of neoplasia such as lung, breast, ovarian, and bladder cancers. Cardiotoxicity is a major concern for patients receiving Dox therapy. Previous work from our laboratory indicated that glucocorticoids (GCs) alleviate Dox-induced apoptosis in cardiomyocytes. Here we have found glucocorticoid-induced leucine zipper (GILZ) to be a mediator of GC-induced cytoprotection. GILZ was found to be induced in cardiomyocytes by GC treatment. Knocking down of GILZ using siRNA resulted in cancelation of GC-induced cytoprotection against apoptosis by Dox treatment. Overexpressing GILZ by transfection was able to protect cells from apoptosis induced by Dox as measured by caspase activation, Annexin V binding and morphologic changes. Western blot analyses indicate that GILZ overexpression prevented cytochrome c release from mitochondria and cleavage of caspase-3. When bcl-2 family proteins were examined, we found that GILZ overexpression causes induction of the pro-survival protein Bcl-xL. Since siRNA against Bcl-xL reverses GC induced cytoprotection, Bcl-xL induction represents an important event in GILZ-induced cytoprotection. Our data suggest that GILZ functions as a cytoprotective gene in cardiomyocytes.
Keywords: Bcl-xL; Caspase-3; Cell death; Chemotherapy; Nuclear receptor; Steroids.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Andeersen CL, Jensen JL, Orntoft TF. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004;64:5245–5250. - PubMed
-
- Ayroldi E, Migliorati G, Bruscoli S, Marchetti C, Zollo O, Cannarile L, D’Adamio F, Riccardi C. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood. 2001;98:743–753. - PubMed
-
- Ayroldi E, Riccardi C. Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 2009;23:3649–3658. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

