Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics
- PMID: 24480314
- PMCID: PMC4847140
- DOI: 10.1016/B978-0-12-800099-1.00003-X
Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics
Abstract
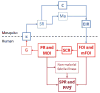
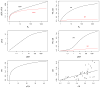
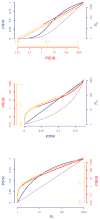
As malaria declines in parts of Africa and elsewhere, and as more countries move towards elimination, it is necessary to robustly evaluate the effect of interventions and control programmes on malaria transmission. To help guide the appropriate design of trials to evaluate transmission-reducing interventions, we review 11 metrics of malaria transmission, discussing their accuracy, precision, collection methods and costs and presenting an overall critique. We also review the nonlinear scaling relationships between five metrics of malaria transmission: the entomological inoculation rate, force of infection, sporozoite rate, parasite rate and the basic reproductive number, R0. Our chapter highlights that while the entomological inoculation rate is widely considered the gold standard metric of malaria transmission and may be necessary for measuring changes in transmission in highly endemic areas, it has limited precision and accuracy and more standardised methods for its collection are required. In areas of low transmission, parasite rate, seroconversion rates and molecular metrics including MOI and mFOI may be most appropriate. When assessing a specific intervention, the most relevant effects will be detected by examining the metrics most directly affected by that intervention. Future work should aim to better quantify the precision and accuracy of malaria metrics and to improve methods for their collection.
Keywords: Elimination; Endemicity; Malaria; Metric; Plasmodium; Transmission; Vector.
© 2014 Elsevier Ltd All rights reserved.
Figures
References
-
- Adungo P, et al. Comparative determination of Plasmodium falciparum sporozoite rates in Afrotropical Anopheles from Kenya by dissection and ELISA. Ann Trop Med Parasitol. 1991;85:387–94. - PubMed
-
- Arnot D. Unstable malaria in Sudan: the influence of the dry season. Clone multiplicity of Plasmodium falciparum infections in individuals exposed to variable levels of disease transmission. Trans R Soc Trop Med Hyg. 1998;92:580–585. - PubMed
-
- Arnot D, et al. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985;230:815–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

