Adipose tissue angiogenesis assay
- PMID: 24480342
- PMCID: PMC4386572
- DOI: 10.1016/B978-0-12-411619-1.00005-7
Adipose tissue angiogenesis assay
Abstract
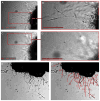
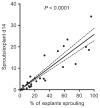
Changes in adipose tissue mass must be accompanied by parallel changes in microcirculation. Investigating the mechanisms that regulate adipose tissue angiogenesis could lead to better understanding of adipose tissue function and reveal new potential therapeutic strategies. Angiogenesis is defined as the formation of new capillaries from existing microvessels. This process can be recapitulated in vitro, by incubation of tissue in extracellular matrix components in the presence of pro-angiogenic factors. Here, we describe a method to study angiogenesis from adipose tissue fragments obtained from mouse and human tissue. This assay can be used to define effects of diverse factors added in vitro, as well as the role of endogenously produced factors on angiogenesis. We also describe approaches to quantify angiogenic potential for the purpose of enabling comparisons between subjects, thus providing information on the role of physiological conditions of the donor on adipose tissue angiogenic potential.
Keywords: Adipocytes; Angiogenesis; Capillary branches; Endothelial cells; Obesity; Sprouts; Vascularization.
© 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Aplin AC, Fogel E, Zorzi P, Nicosia RF. The aortic ring model of angiogenesis. Methods in Enzymology. 2008;443:119–136. - PubMed
-
- Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, et al. Use of the mouse aortic ring assay to study angiogenesis. Nature Protocols. 2012;7:89–104. - PubMed
-
- Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circulation Research. 2007;100:e47–e57. - PubMed
-
- Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Molecular and Cellular Endocrinology. 2010;318:2–9. - PubMed
-
- Crandall DL, Hausman GJ, Kral JG. A review of the of adipose tissue: Anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

