Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation
- PMID: 24480537
- PMCID: PMC4343528
- DOI: 10.1016/j.biomaterials.2014.01.023
Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation
Abstract
Endothelial-targeted delivery of antioxidant enzymes, catalase and superoxide dismutase (SOD), is a promising strategy for protecting organs and tissues from inflammation and oxidative stress. Here we describe Protective Antioxidant Carriers for Endothelial Targeting (PACkET), the first carriers capable of targeted endothelial delivery of both catalase and SOD. PACkET formed through controlled precipitation loaded ~30% enzyme and protected it from proteolytic degradation, whereas attachment of PECAM monoclonal antibodies to surface of the enzyme-loaded carriers, achieved without adversely affecting their stability and functionality, provided targeting. Isotope tracing and microscopy showed that PACkET exhibited specific endothelial binding and internalization in vitro. Endothelial targeting of PACkET was validated in vivo by specific (vs IgG-control) accumulation in the pulmonary vasculature after intravenous injection achieving 33% of injected dose at 30 min. Catalase loaded PACkET protects endothelial cells from killing by H2O2 and alleviated the pulmonary edema and leukocyte infiltration in mouse model of endotoxin-induced lung injury, whereas SOD-loaded PACkET mitigated cytokine-induced endothelial pro-inflammatory activation and endotoxin-induced lung inflammation. These studies indicate that PACkET offers a modular approach for vascular targeting of therapeutic enzymes.
Keywords: Antioxidant enzymes; Inflammation; In vivo vascular targeting; Nanoparticles; Platelet endothelial cellular adhesion molecules.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



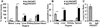
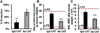
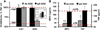
Similar articles
-
Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles.J Control Release. 2010 Aug 17;146(1):144-51. doi: 10.1016/j.jconrel.2010.05.003. Epub 2010 May 18. J Control Release. 2010. PMID: 20483366 Free PMC article.
-
Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates.J Control Release. 2016 Jul 28;234:115-23. doi: 10.1016/j.jconrel.2016.05.040. Epub 2016 May 20. J Control Release. 2016. PMID: 27210108 Free PMC article.
-
Targeted detoxification of selected reactive oxygen species in the vascular endothelium.J Pharmacol Exp Ther. 2009 Nov;331(2):404-11. doi: 10.1124/jpet.109.156877. Epub 2009 Aug 19. J Pharmacol Exp Ther. 2009. PMID: 19692634 Free PMC article.
-
Targeting of superoxide dismutase and catalase to vascular endothelium.J Control Release. 2001 Mar 12;71(1):1-21. doi: 10.1016/s0168-3659(01)00215-2. J Control Release. 2001. PMID: 11245904 Review.
-
Delivery of antioxidant enzyme proteins to the lung.Antioxid Redox Signal. 2001 Feb;3(1):39-62. doi: 10.1089/152308601750100489. Antioxid Redox Signal. 2001. PMID: 11291598 Review.
Cited by
-
Regulation of thrombosis and vascular function by protein methionine oxidation.Blood. 2015 Jun 18;125(25):3851-9. doi: 10.1182/blood-2015-01-544676. Epub 2015 Apr 21. Blood. 2015. PMID: 25900980 Free PMC article. Review.
-
Ex vivo isolated human vessel perfusion system for the design and assessment of nanomedicines targeted to the endothelium.Bioeng Transl Med. 2020 Jan 28;5(2):e10154. doi: 10.1002/btm2.10154. eCollection 2020 May. Bioeng Transl Med. 2020. PMID: 32440561 Free PMC article.
-
Enzymes as Immunotherapeutics.Bioconjug Chem. 2018 Mar 21;29(3):649-656. doi: 10.1021/acs.bioconjchem.7b00719. Epub 2018 Jan 31. Bioconjug Chem. 2018. PMID: 29285931 Free PMC article. Review.
-
Nanotherapies for Treatment of Cardiovascular Disease: A Case for Antioxidant Targeted Delivery.Curr Pathobiol Rep. 2019 Sep;7(3):47-60. doi: 10.1007/s40139-019-00196-4. Epub 2019 Jun 27. Curr Pathobiol Rep. 2019. PMID: 31396435 Free PMC article.
-
Targeting vascular inflammation through emerging methods and drug carriers.Adv Drug Deliv Rev. 2022 May;184:114180. doi: 10.1016/j.addr.2022.114180. Epub 2022 Mar 7. Adv Drug Deliv Rev. 2022. PMID: 35271986 Free PMC article. Review.
References
-
- Attia AB, Yang C, Tan JP, Gao S, Williams DF, Hedrick JL, et al. The effect of kinetic stability on biodistribution and anti-tumor efficacy of drug-loaded biodegradable polymeric micelles. Biomaterials. 2013;34(12):3132–3140. - PubMed
-
- Pangburn TO, Bates FS, Kokkoli E. Polymersomes functionalized via “click” chemistry with the fibronectin mimetic peptides PR_b and GRGDSP for targeted delivery to cells with different levels of alpha 5-beta1 expression. Soft Matter. 2012;8(16):4449–4461.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

