Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants
- PMID: 24480625
- PMCID: PMC3959905
- DOI: 10.1158/0008-5472.CAN-13-0777
Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants
Abstract
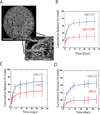
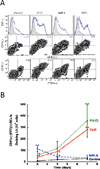
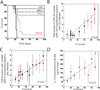
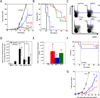
The innate cellular and molecular components required to mediate effective vaccination against weak tumor-associated antigens remain unclear. In this study, we used polymeric cancer vaccines incorporating different classes of adjuvants to induce tumor protection, to identify dendritic cell (DC) subsets and cytokines critical to this efficacy. Three-dimensional, porous polymer matrices loaded with tumor lysates and presenting distinct combinations of granulocyte macrophage colony-stimulating factor (GM-CSF) and various Toll-like receptor (TLR) agonists affected 70% to 90% prophylactic tumor protection in B16-F10 melanoma models. In aggressive, therapeutic B16 models, the vaccine systems incorporating GM-CSF in combination with P(I:C) or CpG-ODN induced the complete regression of solid tumors (≤40 mm(2)), resulting in 33% long-term survival. Regression analysis revealed that the numbers of vaccine-resident CD8(+) DCs, plasmacytoid DCs (pDC), along with local interleukin (IL)-12, and granulocyte colony-stimulating factor (G-CSF) concentrations correlated strongly to vaccine efficacy regardless of adjuvant type. Furthermore, vaccine studies in Batf3(-/-) mice revealed that CD8(+) DCs are required to affect tumor protection, as vaccines in these mice were deficient in cytotoxic T lymphocytes priming and IL-12 induction in comparison with wild-type. These studies broadly demonstrate that three-dimensional polymeric vaccines provide a potent platform for prophylactic and therapeutic protection, and can be used as a tool to identify critical components of a desired immune response. Specifically, these results suggest that CD8(+) DCs, pDCs, IL-12, and G-CSF play important roles in priming effective antitumor responses with these vaccines.
©2014 AACR.
Conflict of interest statement
Figures


References
-
- Lanzavecchia A, Sallusto F. Regulation of T Cell Immunity by dendritic cells. Cell. 2001;106:263–266. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. - PubMed
-
- Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat. Immunol. 2006;7:1237–1242. - PubMed
-
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

