SHP2E76K mutant promotes lung tumorigenesis in transgenic mice
- PMID: 24480804
- PMCID: PMC4123642
- DOI: 10.1093/carcin/bgu025
SHP2E76K mutant promotes lung tumorigenesis in transgenic mice
Abstract
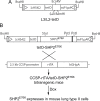
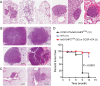
Lung cancer is a major disease carrying heterogeneous molecular lesions and many of them remain to be analyzed functionally in vivo. Gain-of-function (GOF) SHP2 (PTPN11) mutations have been found in various types of human cancer, including lung cancer. However, the role of activating SHP2 mutants in lung cancer has not been established. We generated transgenic mice containing a doxycycline (Dox)-inducible activating SHP2 mutant (tetO-SHP2(E76K)) and analyzed the role of SHP2(E76K) in lung tumorigenesis in the Clara cell secretory protein (CCSP)-reverse tetracycline transactivator (rtTA)/tetO-SHP2(E76K) bitransgenic mice. SHP2(E76K) activated Erk1/Erk2 (Erk1/2) and Src, and upregulated c-Myc and Mdm2 in the lungs of bitransgenic mice. Atypical adenomatous hyperplasia and small adenomas were observed in CCSP-rtTA/tetO-SHP2(E76K) bitransgenic mice induced with Dox for 2-6 months and progressed to larger adenoma and adenocarcinoma by 9 months. Dox withdrawal from bitransgenic mice bearing magnetic resonance imaging-detectable lung tumors resulted in tumor regression. These results show that the activating SHP2 mutant promotes lung tumorigenesis and that the SHP2 mutant is required for tumor maintenance in this mouse model of non-small cell lung cancer. SHP2(E76K) was associated with Gab1 in the lung of transgenic mice. Elevated pGab1 was observed in the lung of Dox-induced CCSP-rtTA/tetO-SHP2(E76K) mice and in cell lines expressing SHP2(E76K), indicating that the activating SHP2 mutant autoregulates tyrosine phosphorylation of its own docking protein. Gab1 tyrosine phosphorylation is sensitive to inhibition by the Src inhibitor dasatinib in GOF SHP2-mutant-expressing cells, suggesting that Src family kinases are involved in SHP2 mutant-induced Gab1 tyrosine phosphorylation.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Tonks N.K. (2006). Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol., 7, 833–846 - PubMed
-
- Julien S.G., et al. (2011). Inside the human cancer tyrosine phosphatome. Nat. Rev. Cancer, 11, 35–49 - PubMed
-
- Xu W., et al. (1999). Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell, 3, 629–638 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

