Quality-adjusted survival following treatment of malignant pleural effusions with indwelling pleural catheters
- PMID: 24480929
- PMCID: PMC4694176
- DOI: 10.1378/chest.13-1908
Quality-adjusted survival following treatment of malignant pleural effusions with indwelling pleural catheters
Abstract
Background: Malignant pleural effusions (MPEs) are a frequent cause of dyspnea in patients with cancer. Although indwelling pleural catheters (IPCs) have been used since 1997, there are no studies of quality-adjusted survival following IPC placement.
Methods: With a standardized algorithm, this prospective observational cohort study of patients with MPE treated with IPCs assessed global health-related quality of life using the SF-6D to calculate utilities. Quality-adjusted life days (QALDs) were calculated by integrating utilities over time.
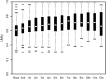
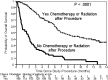
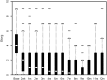
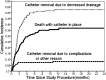
Results: A total of 266 patients were enrolled. Median quality-adjusted survival was 95.1 QALDs. Dyspnea improved significantly following IPC placement (P < .001), but utility increased only modestly. Patients who had chemotherapy or radiation after IPC placement (P < .001) and those who were more short of breath at baseline (P = .005) had greater improvements in utility. In a competing risk model, the 1-year cumulative incidence of events was death with IPC in place, 35.7%; IPC removal due to decreased drainage, 51.9%; and IPC removal due to complications, 7.3%. Recurrent MPE requiring repeat intervention occurred in 14% of patients whose IPC was removed. Recurrence was more common when IPC removal was due to complications (P = .04) or malfunction (P < .001) rather than to decreased drainage.
Conclusions: IPC placement has significant beneficial effects in selected patient populations. The determinants of quality-adjusted survival in patients with MPE are complex. Although dyspnea is one of them, receiving treatment after IPC placement is also important. Future research should use patient-centered outcomes in addition to time-to-event analysis.
Trial registry: ClinicalTrials.gov; No.: NCT01117740; URL: www.clinicaltrials.gov.
Figures


References
-
- Haas AR, Sterman DH, Musani AI. Malignant pleural effusions: management options with consideration of coding, billing, and a decision approach. Chest. 2007;132(3):1036-1041. - PubMed
-
- Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5_suppl):e455S-497S. - PubMed
-
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389. - PubMed
-
- Putnam JB, Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86(10):1992-1999. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

