Denosumab should be the treatment of choice for bisphosphonate refractory hypercalcaemia of malignancy
- PMID: 24481018
- PMCID: PMC3912364
- DOI: 10.1136/bcr-2013-202861
Denosumab should be the treatment of choice for bisphosphonate refractory hypercalcaemia of malignancy
Abstract
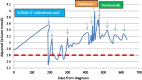
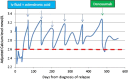
Denosumab, a fully humanised monoclonal antibody, is licensed for treatment of postmenopausal osteoporosis, hormone ablation-induced bone loss and for prevention of skeleton-related events in patients with bone metastases from solid tumours. In pivotal phase 3 randomised trials, denosumab caused profound hypocalcaemia in patients with normocalcaemia despite oral calcium and vitamin D supplementation. This significant hypocalcaemic effect can be exploited to treat hypercalcaemia of malignancy (HCM). Recent reports from the USA suggest that denosumab is an effective treatment of HCM. According to our knowledge, we report the first two cases in UK with bisphosphonate refractory hypercalcaemia who responded to denosumab injections. Our first case gained 7 months of stabilisation of hypercalcaemia following prolonged admissions with life-threatening levels, while our second case achieved rapid normalisation of serum calcium levels for the first time in 14 months. We conclude that denosumab should be the treatment of choice for patients with bisphosphonate refractory hypercalcaemia.
Figures
Similar articles
-
Denosumab for the treatment of bisphosphonate-refractory hypercalcemia.J Clin Oncol. 2012 Oct 10;30(29):e299. doi: 10.1200/JCO.2012.41.7923. Epub 2012 Sep 4. J Clin Oncol. 2012. PMID: 22949145 No abstract available.
-
Use of denosumab for renal cell carcinoma-associated malignant hypercalcemia: a case report and review of the literature.Clin Genitourin Cancer. 2013 Dec;11(4):e24-6. doi: 10.1016/j.clgc.2013.06.002. Epub 2013 Sep 2. Clin Genitourin Cancer. 2013. PMID: 24007982 No abstract available.
-
Denosumab for patients with persistent or relapsed hypercalcemia of malignancy despite recent bisphosphonate treatment.J Natl Cancer Inst. 2013 Sep 18;105(18):1417-20. doi: 10.1093/jnci/djt225. Epub 2013 Aug 29. J Natl Cancer Inst. 2013. PMID: 23990665 Free PMC article. Clinical Trial.
-
[Inhibition of RANK ligand to treat bone metastases].Bull Cancer. 2013 Nov;100(11):1207-13. doi: 10.1684/bdc.2013.1835. Bull Cancer. 2013. PMID: 24158618 Review. French.
-
[Bone and calcium metabolism associated with malignancy. Malignancy-associated hypercalcemia.].Clin Calcium. 2018;28(11):1503-1508. Clin Calcium. 2018. PMID: 30374007 Review. Japanese.
Cited by
-
HTLV-1, ATLL, severe hypercalcaemia and HIV-1 co-infection: an overview.Pan Afr Med J. 2018 May 28;30:61. doi: 10.11604/pamj.2018.30.61.13238. eCollection 2018. Pan Afr Med J. 2018. PMID: 30344845 Free PMC article. Review.
-
Hypercalcemia of Malignancy: An Update on Pathogenesis and Management.N Am J Med Sci. 2015 Nov;7(11):483-93. doi: 10.4103/1947-2714.170600. N Am J Med Sci. 2015. PMID: 26713296 Free PMC article. Review.
-
Rare and changeable as a chameleon: paraneoplastic syndromes in renal cell carcinoma.World J Urol. 2018 Jun;36(6):849-854. doi: 10.1007/s00345-018-2215-9. Epub 2018 Feb 10. World J Urol. 2018. PMID: 29429069 Review.
-
Parathyroid hormone-related protein levels and treatment outcomes in hypercalcemia of malignancy: a retrospective cohort study.JBMR Plus. 2025 Jan 15;9(3):ziae178. doi: 10.1093/jbmrpl/ziae178. eCollection 2025 Mar. JBMR Plus. 2025. PMID: 39925622 Free PMC article.
-
Denosumab is Effective for Controlling Serum Calcium Levels in Patients with Humoral Hypercalcemia of Malignancy Syndrome: A Case Report on Parathyroid Hormone-related Protein-producing Cholangiocarcinoma.Intern Med. 2016;55(23):3453-3457. doi: 10.2169/internalmedicine.55.7134. Epub 2016 Dec 1. Intern Med. 2016. PMID: 27904108 Free PMC article.
References
-
- National Cancer Institute: PDQ Hypercalcemia Bethesda, MD: National Cancer Institute; Date last modified 01/09/2013. [Internet]. [cited 28 Oct 2013]. http://www.cancer.gov/cancertopics/pdq/supportivecare/hypercalcemia/Heal...
-
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001;19:558–67 - PubMed
-
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
