Stress and sex in malaria parasites: Why does commitment vary?
- PMID: 24481194
- PMCID: PMC3854026
- DOI: 10.1093/emph/eot011
Stress and sex in malaria parasites: Why does commitment vary?
Abstract
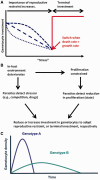
For vector-borne parasites such as malaria, how within- and between-host processes interact to shape transmission is poorly understood. In the host, malaria parasites replicate asexually but for transmission to occur, specialized sexual stages (gametocytes) must be produced. Despite the central role that gametocytes play in disease transmission, explanations of why parasites adjust gametocyte production in response to in-host factors remain controversial. We propose that evolutionary theory developed to explain variation in reproductive effort in multicellular organisms, provides a framework to understand gametocyte investment strategies. We examine why parasites adjust investment in gametocytes according to the impact of changing conditions on their in-host survival. We then outline experiments required to determine whether plasticity in gametocyte investment enables parasites to maintain fitness in a variable environment. Gametocytes are a target for anti-malarial transmission-blocking interventions so understanding plasticity in investment is central to maximizing the success of control measures in the face of parasite evolution.
Keywords: Plasmodium; commitment; gametocyte; phenotypic plasticity; stress; transmission.
Figures
References
-
- Garnham PCC. Malaria Parasites and other Haemosproridia. Oxford: Blackwell Science; 1966.
-
- Baton LA, Ranford-Cartwright LC. Spreading the seeds of million murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–80. - PubMed
-
- Buckling A, Ranford-Cartwright LC, Miles A, et al. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology. 1999;118:339–46. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

